Key Points
DNM2-dependent endocytosis in MKs regulates megakaryopoiesis, thrombopoiesis, and bone marrow homeostasis.
Abstract
Dynamins are highly conserved large GTPases (enzymes that hydrolyze guanosine triphosphate) involved in endocytosis and vesicle transport, and mutations in the ubiquitous and housekeeping dynamin 2 (DNM2) have been associated with thrombocytopenia in humans. To determine the role of DNM2 in thrombopoiesis, we generated Dnm2fl/flPf4-Cre mice specifically lacking DNM2 in the megakaryocyte (MK) lineage. Dnm2fl/flPf4-Cre mice had severe macrothrombocytopenia with moderately accelerated platelet clearance. Dnm2-null bone marrow MKs had altered demarcation membrane system formation in vivo due to defective endocytic pathway, and fetal liver–derived Dnm2-null MKs formed proplatelets poorly in vitro, showing that DNM2-dependent endocytosis plays a major role in MK membrane formation and thrombopoiesis. Endocytosis of the thrombopoietin receptor Mpl was impaired in Dnm2-null platelets, causing constitutive phosphorylation of the tyrosine kinase JAK2 and elevated circulating thrombopoietin levels. MK-specific DNM2 deletion severely disrupted bone marrow homeostasis, as reflected by marked expansion of hematopoietic stem and progenitor cells, MK hyperplasia, myelofibrosis, and consequent extramedullary hematopoiesis and splenomegaly. Taken together, our data demonstrate that unrestrained MK growth and proliferation results in rapid myelofibrosis and establishes a previously unrecognized role for DNM2-dependent endocytosis in megakaryopoiesis, thrombopoiesis, and bone marrow homeostasis.
Introduction
Blood platelets are produced in the bone marrow by megakaryocytes (MKs) in a process that requires the production of pseudopodial projections, termed proplatelets, that extend through the vascular sinusoids and release platelets into the bloodstream.1,2 This process requires extensive intracellular membrane rearrangements in MKs, including the formation of the demarcation membrane system (DMS), a surface-connected membrane extension that invaginates into the cell body and further develops to provide membranes necessary for platelet formation.3-5 The mechanisms responsible for these membrane rearrangements remain poorly understood.
Dynamins (DNMs) are highly conserved large GTPases involved in a wide range of cellular functions, including endocytosis and vesicle transport.6 Particularly, DNMs are involved in the GTPase-dependent fission of vesicles generated from the plasma membrane by clathrin-dependent and -independent mechanisms. The classical DNM family is composed of 3 members: DNM1, DNM2, and DNM3. The housekeeping DNM2 is ubiquitously expressed and regulates both receptor-mediated endocytosis and vesicle transport between endomembrane system compartments.7-11 DNM1 and DNM3 are mainly expressed in the brain and have specialized endocytic roles such as presynaptic vesicle recycling.12-14
Human platelets express all 3 classical DNMs, whereas mouse platelets predominantly express DNM2.15,16 Previous studies have investigated the role of DNMs, particularly DNM3, in MK maturation and platelet formation. DNM3 expression increases during maturation of cultured human MKs, and its knockdown by RNA interference inhibits MK growth and development.17,18 However, individuals expressing a truncated and inactive DNM3 in MKs have normal blood platelet counts and minimally affected mean platelet volumes, indicating that DNM3 plays a redundant role in platelet formation in humans.19 In contrast, mutations in the housekeeping DNM2 have been associated with thrombocytopenia and hematopoietic disorders such as neutropenia and early T-cell precursor acute lymphoblastic leukemia.20-23
We investigated the role of DNM2 in MK maturation and platelet formation by generating Dnm2fl/flPf4-Cre mice specifically lacking DNM2 in the MK lineage via Cre-loxP recombination. Dnm2fl/flPf4-Cre mice had severe macrothrombocytopenia with moderately accelerated platelet clearance. Dnm2-null bone marrow MKs had impaired DMS formation in vivo due to defective endocytic pathway, and fetal liver–derived Dnm2-null MKs formed proplatelets poorly in vitro. Endocytosis of the thrombopoietin (TPO) receptor Mpl was impaired in Dnm2-null platelets, causing constitutive phosphorylation of the tyrosine kinase JAK2 and elevated circulating TPO levels. Importantly, Dnm2fl/flPf4-Cre mice developed expansion of hematopoietic stem and progenitor cell (HSPC) compartment, MK hyperplasia, myelofibrosis (MF) and consequent extramedullary hematopoiesis (EMH), and splenomegaly. Taken together, our data demonstrate that unrestrained MK growth and proliferation results in rapid MF and establishes a novel role for DNM2-dependent endocytosis in megakaryopoiesis, thrombopoiesis, and bone marrow homeostasis.
Methods
Mice
Dnm2fl/fl mice carrying loxP sites on either side of Dnm2 exon 2 were paired with Pf4-Cre mice to delete Dnm2 in the MK lineage.8,24 All studies were performed on 7- to 10-week-old C57BL/6 Dnm2fl/fl and Dnm2fl/flPf4-Cre mice. Mice were treated according to the National Institutes of Health and Boston Children’s Hospital Animal Care and Use Committee guidelines.
Complete blood counts and platelet preparation
Mouse blood was collected from retroorbital plexus in EDTA and anticoagulant citrate dextrose solution for complete blood counts and platelet preparation, respectively. Complete blood counts were determined using a HEMAVET 950 automated hematologic analyzer (Drew Scientific).25 Platelet-rich plasma was obtained by centrifugation of the blood at 100g for 8 minutes, followed by centrifugation of the supernatant and the buffy coat at 100g for 6 minutes. After washing twice in 140 mM sodium chloride (NaCl), 5 mM potassium chloride, 12 mM trisodium citrate, 10 mM glucose, and 12.5 mM sucrose, pH 6.0, platelets were resuspended in 140 mM NaCl, 3 mM potassium chloride, 0.5 mM magnesium chloride, 5 mM sodium bicarbonate, 10 mM glucose, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, and counted by flow cytometry using 5.5-µm diameter SPHERO rainbow beads (Spherotech) as reference.25,26
Immunoblot analysis
Platelets were lysed in 1% Nonidet P-40, 150 mM NaCl, and 50 mM tris(hydroxymethyl)aminomethane/hydrochloric acid, pH 7.4, containing 1 mM EGTA, 1 mM sodium orthovanadate, and cOmplete Protease Inhibitor Cocktail (Roche). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) buffer was added to lysates in the presence of 5% β-mercaptoethanol. Proteins were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membrane. After blocking overnight with 1% bovine serum albumin (BSA) in 0.2% Tween-20, 100 mM NaCl, and 20 mM tris(hydroxymethyl)aminomethane/hydrochloric acid, pH 7.4, membranes were probed with rabbit antibodies directed against DNM2, Mpl (Abcam), actin (Sigma-Aldrich), JAK2, or phosphorylated JAK2 (Tyr1007/1008; EMD Millipore), followed by secondary horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin (Ig)G antibody (Thermo Fisher Scientific). Detection was performed by enhanced chemiluminescence.25,26
Flow cytometry
Platelet surface glycoproteins (GPs) were investigated by flow cytometry using fluorescently conjugated antibodies: GPIbα, GPIbβ, GPIX, GPV, GPVI (EMFRET Analytics), and GPIIIa (BD Biosciences). For Mpl surface expression, platelets were incubated with a rabbit antibody directed against the extracellular domain of Mpl (a gift from Dr Wei Tong, University of Pennsylvania) or control rabbit IgG (Santa Cruz Biotechnology), followed by secondary Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Invitrogen). Fluorescence was quantified using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences). A total of 20 000 events were analyzed for each sample.25,26
Platelet survival
Endogenous platelet survival was determined by intravenous injection of biotin- N-hydroxysuccinimide in mice.27 Blood was collected by retroorbital bleed, and the percentage of biotin-positive platelets was determined by flow cytometry using phycoerythrin-labeled streptavidin. The number of phycoerythrin-positive platelets at 5 minutes was normalized to 100%.
Laser fluorescence spinning disk confocal microscopy
Platelets were fixed by adding equal volumes of 8% paraformaldehyde in phosphate-buffered saline (PBS) to platelet-rich plasma for 15 minutes at room temperature.28 Cells were pelleted by centrifugation, washed with PBS, and resuspended in PBS plus 1% BSA prior to spotting on coverslips (thickness No. 1.5) and incubation for 90 minutes at 37°C with saturating humidity. Platelet preparations were rinsed with PBS and blocked for 60 minutes with hybridization buffer (0.2% Triton X-100 in PBS plus 1% BSA and 2% donkey serum); the same buffer was used for incubation with antibodies directed against GPIbα (EMFRET Analytics) and P-selectin (Santa Cruz Biotechnology). After washing, preparations were incubated for 60 minutes with fluorescently labeled donkey antibodies specific for primary antibody species (Invitrogen), washed, post-fixed, and mounted on slides with fluorescent mounting medium (Dako). Preparations were imaged with a ×63 Olympus oil-immersion objective (1.4 NA) on an Olympus 1X81 inverted fluorescence microscope equipped with an Improvision Piezo focus drive (250-nm stepping), ×1.5 magnification lens (Spectral Applied Research), separate diode-pumped solid-state laser lines, a spinning disk confocal scan head (Quorum Technologies), and a Hamamatsu EM Back-Thinned electron-multiplying charge-coupled device camera (512 × 512 pixels). Volocity (version 6.0+) software was used for image acquisition and subsequent processing (deconvolution and rendering); images were exported to Adobe Photoshop for final presentation.
Transmission electron microscopy
Bone marrow cells were obtained by flushing mouse femurs with 2.5% glutaraldehyde in PBS and fixed overnight. Subsequently, bone marrow cells were post-fixed with 2% osmium tetroxide in water for 60 minutes and dehydrated in a graded series of acetone before embedding in Epon-Araldite. Thin sections were cut and stained with uranyl acetate and lead citrate. Grids were examined with a JEOL JEM-1011 electron microscope at 80 kV. Images were captured with a side-mounted high-resolution charge-coupled device camera (Advantage; Advanced Microscopy Techniques).28
Mouse bone marrow MK preparation
Flushed bone marrow cells were cultured in 2.6% serum-supplemented StemPro-34 medium (Gibco) with 2 mM l-glutamine, penicillin/streptomycin, and 50 ng/mL of recombinant stem cell factor (SCF; R&D Systems) for 2 days.29 Cells were then cultured for 2 days in the presence of 50 ng/mL of SCF and 50 ng/mL of recombinant TPO (R&D Systems), and cultured for 2 additional days in the presence of TPO only. Mature MKs were enriched over BSA gradient, fixed in 4% paraformaldehyde, and centrifuged onto poly-l-lysine–coated coverslips. After washing in PBS, cells were permeabilized with 0.1% Triton X-100/PBS and blocked in 5% goat serum/PBS. Cells were incubated with a mouse anti-α-tubulin antibody (Sigma-Aldrich) or rabbit antibodies directed against clathrin heavy chain, early endosome antigen 1 (EEA1), calreticulin (Cell Signaling Technology), and APPL1 (Abcam), and probed with fluorescently labeled antibodies (Invitrogen). Coverslips were mounted onto microscope slides with AquaMount (Polysciences). Images were taken on a Leica SP5 confocal microscope.25
Mouse fetal liver–derived MK preparation
Mouse embryonic fetal livers were isolated 14.5 days postcoitus and homogenized in Dulbecco’s modified Eagle medium (Gibco) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 1% penicillin/streptomycin (Gibco), and 50 ng/mL of TPO.25 Cells were cultured at 37°C and 5% carbon dioxide. Mature MKs were enriched using a BSA density gradient on day 3. MK cultures were incubated for an additional 18 hours to induce proplatelet formation and the release of nascent platelets. The percentage of MKs forming proplatelets was determined by counting proplatelet-forming and total MKs using an Olympus CKX41 microscope.
Mouse bone marrow and spleen histology
Femurs and spleens of Dnm2fl/fl and Dnm2fl/flPf4-Cre mice were fixed overnight in 4% paraformaldehyde/PBS.25 Bones were decalcified in 0.5 M EDTA, pH 8.0, (Boston BioProducts) for 7 days under rotation, exchanging EDTA twice daily. Tissues were paraffin embedded, and sections were stained with hematoxylin and eosin (H&E) or transforming growth factor-β1 (TGF-β1). Bone marrow reticulin staining was performed at the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core Facility.
MK ploidy
Bone marrow cells were flushed with Hanks balanced salt solution containing 0.38% sodium citrate, 1 mM adenosine, 1 mM theophylline, and 5% FBS (washing buffer); and erythrocytes were lysed with 0.15 M ammonium chloride, 10 mM potassium bicarbonate, and 0.1 mM disodium EDTA, pH 7.4. Bone marrow cells were fixed in 70% ethanol, washed twice, and resuspended in washing buffer. Cells were incubated with fluorescein isothiocyanate–conjugated rat anti-CD41 antibody or isotype control (BD Biosciences). After washing twice, cells were incubated with 0.5 mg/mL of RNase (Sigma-Aldrich), incubated with 50 µg/mL of propidium iodide, and analyzed by flow cytometry.30
Colony forming units, TGF-β1, and TPO levels
For multipotential hematopoietic progenitor colony forming units (CFUs), 1 × 105 bone marrow or spleen cells isolated from Dnm2fl/fl and Dnm2fl/flPf4-Cre were diluted into a semisolid methylcellulose-based medium (STEMCELL Technologies) and cultured in 35-mm culture dishes in the presence of 10 ng/mL of interleukin (IL)-3, 10 ng/mL of IL-6, 50 ng/mL of SCF, and 3 U/mL of erythropoietin. Total number of colonies was counted after 7 days of culture. For MK-specific CFUs, 5 × 105 bone marrow cells from Dnm2fl/fl and Dnm2fl/flPf4-Cre were plated into MegaCult collagen-based medium (STEMCELL Technologies) and cultured for 7 days in the presence of 10 ng/mL of IL-3, 20 ng/mL of IL-6, and 50 ng/mL of TPO (R&D Systems). Cells were dehydrated and fixed in acetone and stained for acetylcholinesterase activity. Colonies containing at least 3 acetylcholinesterase-positive cells were scored.31
Mouse femurs were flushed with 1 mL of PBS and centrifuged. TGF-β1 levels were measured in supernatant after acid treatment using a Human/Mouse TGF-β1 ELISA Ready-SET-Go! kit (eBioscience). Plasma TPO levels were quantified using a Mouse Thrombopoietin Quantikine ELISA kit (R&D Systems).
HSPC analysis
Bone marrow or spleen cells were collected and prepared for staining by erythrocyte lysis (BD Pharm Lyse; BD Biosciences) and homogenization through a 70-µm filter. Cells were stained in ice-cold PBS containing 2% FBS using the following antibodies (eBioscience): lineage cocktail containing CD3ε, CD5, Ter-119, Gr-1, Mac-1, and B220; Kit, Sca-1, CD150, CD48, CD34, CD16/32, CD41, and CD105.32,33 4′,6 Diamidino-2-phenylindole (Invitrogen) was used for dead cell discrimination. Samples were analyzed by flow cytometry using an LSR II (BD Biosciences). Postacquisition analysis of data was performed with FlowJo software V9.2.3.
Statistical analysis
All experiments were performed at least in triplicate, and data are represented as mean ± standard error of the mean (SEM). Data were analyzed by Student t test using Prism software (GraphPad). Differences were considered significant when P values were <.05.
Results
Macrothrombocytopenia in Dnm2fl/flPf4-Cre mice
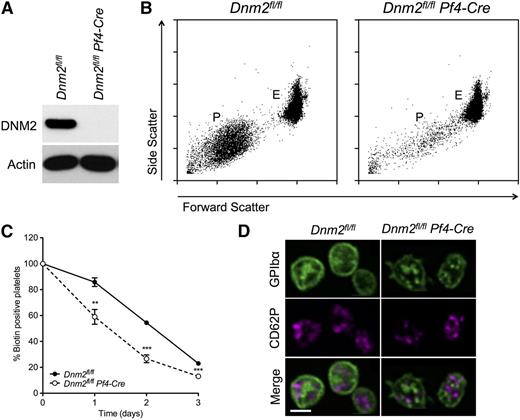
Ubiquitous Dnm2 deficiency in mice causes lethality before embryonic day 8.5.8 Dnm2fl/fl mice carrying loxP sites on either side of Dnm2 exon 2 were therefore paired with Pf4-Cre mice to specifically delete Dnm2 in the MK lineage.24 Dnm2fl/flPf4-Cre mice were viable, fertile, and obtained with a normal Mendelian inheritance ratio (data not shown). As expected, Dnm2fl/flPf4-Cre platelets lacked DNM2, a protein of 105 kDa in Dnm2fl/fl platelets (Figure 1A).
Macrothrombocytopenia in Dnm2fl/flPf4-Cre mice. (A) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelet lysates corresponding to 2 µg of protein were subjected to SDS-PAGE and probed for DNM2 and actin as indicated. (B) Blood was collected from Dnm2fl/fl and Dnm2fl/flPf4-Cre mice and analyzed by flow cytometry. Results are forward/side scatter dot plots and are representative of 4 independent experiments. P indicates platelets; E, erythrocytes. (C) Endogenous platelet survival measured by in vivo biotinylation. (D) High-resolution laser fluorescence spinning disk confocal microscopy imaging of GPIbα and P-selectin (CD62P) in fixed and permeabilized platelets. Bar represents 2 µm.
Macrothrombocytopenia in Dnm2fl/flPf4-Cre mice. (A) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelet lysates corresponding to 2 µg of protein were subjected to SDS-PAGE and probed for DNM2 and actin as indicated. (B) Blood was collected from Dnm2fl/fl and Dnm2fl/flPf4-Cre mice and analyzed by flow cytometry. Results are forward/side scatter dot plots and are representative of 4 independent experiments. P indicates platelets; E, erythrocytes. (C) Endogenous platelet survival measured by in vivo biotinylation. (D) High-resolution laser fluorescence spinning disk confocal microscopy imaging of GPIbα and P-selectin (CD62P) in fixed and permeabilized platelets. Bar represents 2 µm.
Dnm2fl/flPf4-Cre mice developed severe macrothrombocytopenia (Figure 1B; Table 1). However, the clearance of Dnm2-null platelets was moderately accelerated in vivo, because the platelet half-life was 38.4 ± 0.9 hours in Dnm2fl/flPf4-Cre mice compared with 48.1 ± 0.6 hours in Dnm2fl/fl mice (mean ± SEM; n = 5 in each group; P < .0001), a 20% decrease (Figure 1C). Dnm2fl/flPf4-Cre mice also had mild lymphocytopenia and slightly increased granulocyte and monocyte counts but normal erythrocyte counts (Table 1).
Complete blood counts in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice
| Parameter . | Dnm2fl/fl . | Dnm2fl/flPf4-Cre . | P . |
|---|---|---|---|
| Erythrocytes (103/µL) | 9376 ± 347 | 8997 ± 747 | NS |
| MCV (fL) | 48.14 ± 3.36 | 48.30 ± 3.42 | NS |
| Platelets (103/µL) | 816 ± 82 | 274 ± 123 | <.0001 |
| MPV (fL) | 4.78 ± 0.24 | 7.28 ± 0.50 | <.0001 |
| Lymphocytes (103/µL) | 8.25 ± 1.14 | 6.09 ± 1.88 | .0025 |
| Granulocytes (103/µL) | 2.01 ± 0.34 | 2.59 ± 0.52 | .0036 |
| Monocytes (103/µL) | 0.40 ± 0.08 | 0.54 ± 0.17 | .0184 |
| Parameter . | Dnm2fl/fl . | Dnm2fl/flPf4-Cre . | P . |
|---|---|---|---|
| Erythrocytes (103/µL) | 9376 ± 347 | 8997 ± 747 | NS |
| MCV (fL) | 48.14 ± 3.36 | 48.30 ± 3.42 | NS |
| Platelets (103/µL) | 816 ± 82 | 274 ± 123 | <.0001 |
| MPV (fL) | 4.78 ± 0.24 | 7.28 ± 0.50 | <.0001 |
| Lymphocytes (103/µL) | 8.25 ± 1.14 | 6.09 ± 1.88 | .0025 |
| Granulocytes (103/µL) | 2.01 ± 0.34 | 2.59 ± 0.52 | .0036 |
| Monocytes (103/µL) | 0.40 ± 0.08 | 0.54 ± 0.17 | .0184 |
Results are expressed as mean ± SEM (n = 12 in each group).
MCV, mean corpuscular volume; MPV, mean platelet volume; NS, not significant.
GP expression and morphology of Dnm2-null platelets
Expression of major GPs on the surface of Dnm2-null platelets was examined by flow cytometry (Table 2). Expression of the fibrinogen receptor subunit, integrin β3, was doubled in Dnm2-null platelets compared with that of control platelets, reflecting their enlarged size. Expression of the von Willebrand factor receptor complex subunits (GPIbα, GPIbβ, GPV, and GPIX), however, was normal or increased, whereas that of the collagen receptor GPVI was decreased.
Expression of major surface GPs on Dnm2-null platelets
| Platelet GP . | Dnm2fl/fl . | Dnm2fl/flPf4-Cre . | P . |
|---|---|---|---|
| GPIbα (CD42b) | 149 ± 13 | 153 ± 10 | NS |
| GPIbβ (CD42c) | 462 ± 16 | 591 ± 34 | .0094 |
| GPIX (CD42a) | 101 ± 19 | 177 ± 21 | .0262 |
| GPV (CD42d) | 85 ± 1 | 79 ± 3 | NS |
| GPIIIa (CD61, integrin β3) | 142 ± 7 | 289 ± 6 | <.0001 |
| GPVI | 34 ± 2 | 21 ± 1 | .0014 |
| Platelet GP . | Dnm2fl/fl . | Dnm2fl/flPf4-Cre . | P . |
|---|---|---|---|
| GPIbα (CD42b) | 149 ± 13 | 153 ± 10 | NS |
| GPIbβ (CD42c) | 462 ± 16 | 591 ± 34 | .0094 |
| GPIX (CD42a) | 101 ± 19 | 177 ± 21 | .0262 |
| GPV (CD42d) | 85 ± 1 | 79 ± 3 | NS |
| GPIIIa (CD61, integrin β3) | 142 ± 7 | 289 ± 6 | <.0001 |
| GPVI | 34 ± 2 | 21 ± 1 | .0014 |
Platelets from control Dnm2fl/fl and Dnm2fl/flPf4-Cre mice were incubated with fluorescently labeled antibodies directed against the indicated platelet GPs. Data are expressed as mean fluorescence intensity and represent mean ± SEM (n = 5 in each group).
NS, not significant.
Dnm2-null platelets showed decreased and less well defined distribution of surface and internal membrane GPIbα, a marker of the platelet open canalicular system, as evidenced by high-resolution immunofluorescence confocal microscopy (Figure 1D). The distribution of α-granules, as evaluated by intracellular P-selectin staining, appeared normal within Dnm2-null platelets.
Altered ultrastructure of Dnm2-null bone marrow MKs
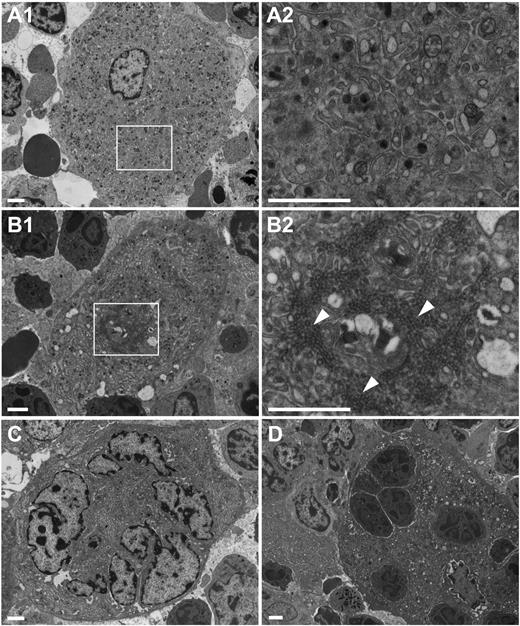
The significant decrease in blood platelet counts, despite moderate increased platelet clearance, prompted us to examine the ultrastructure of Dnm2fl/flPf4-Cre bone marrow MKs. Control Dnm2fl/fl MKs formed the DMS (Figure 2A), the highly organized intracellular membrane structure and membrane reservoir for future platelets that is associated with late-stage mature MKs.3-5 Although present, the DMS was dramatically altered in Dnm2fl/flPf4-Cre MKs (Figure 2B-C), appearing at times as a compact, narrow twisting membrane system of clathrin-coated vesicles (Figure 2B, arrowheads), resembling the “clathrin-coated profiles” of Dnm1-null inhibitory synapses.12,13 Emperipolesis, or the presence of intact nucleated cells within the cytoplasm, was also a common feature of bone marrow Dnm2fl/flPf4-Cre MKs (Figure 2D).
Altered bone marrow Dnm2fl/flPf4-Cre MK ultrastructure. Transmission electron microscopy analysis of bone marrow Dnm2fl/fl MKs (A) and Dnm2fl/flPf4-Cre MKs (B-D). Areas within boxes in panels A1 and B1 are shown at higher magnification in panels A2 and B2, respectively. Arrowheads point to clathrin-coated vesicles. Bars represent 2 µm.
Altered bone marrow Dnm2fl/flPf4-Cre MK ultrastructure. Transmission electron microscopy analysis of bone marrow Dnm2fl/fl MKs (A) and Dnm2fl/flPf4-Cre MKs (B-D). Areas within boxes in panels A1 and B1 are shown at higher magnification in panels A2 and B2, respectively. Arrowheads point to clathrin-coated vesicles. Bars represent 2 µm.
Impaired endocytic pathway in Dnm2-null bone marrow MKs
DNM2 plays a critical role in clathrin-dependent receptor-mediated endocytosis.9-11 We therefore investigated the fate of the endocytic pathway in cultured bone marrow MKs (Figure 3A). Clathrin heavy chain was punctate in control MKs, consistent with continuous endocytosis, whereas it appeared abnormally clustered in the center of the cell in the absence of DNM2. The early endosome markers EEA1 and APPL1,34,35 which stained large vesicle structures in control MKs, were mislocalized in Dnm2-null MKs. In contrast, the distribution of the endoplasmic reticulum chaperone calreticulin36 was normal in Dnm2-null MKs. Taken together, the data show that loss of DNM2 predominantly impairs the endocytic pathway in MKs but apparently has a lesser effect on the exocytic pathway, as indicated by calreticulin staining.
Impaired endocytic pathway and proplatelet formation in Dnm2-null MKs. (A) Representative clathrin heavy chain, EEA1, APPL1, and calreticulin immunofluorescence images of Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrow MKs. Bars represent 10 µm. (B) Dnm2fl/fl (n = 10) and Dnm2fl/flPf4-Cre (n = 16) fetal liver–derived MKs extending proplatelets were counted 18 hours after BSA gradient (day 4 of culture) and expressed as percentage of total MKs (mean ± SEM; ***P < .0001). Representative light microscopy images of fetal liver–derived Dnm2fl/fl (C) and Dnm2fl/flPf4-Cre (D) MK-forming proplatelets in vitro. Areas within boxes in panels C1 and D1 are shown at higher magnification in panels C2 and D2, respectively. Bars represent 50 µm. Representative α-tubulin immunofluorescence images of fetal liver–derived Dnm2fl/fl (E) and Dnm2fl/flPf4-Cre (F) MK-forming proplatelets in vitro. Bars represent 20 µm.
Impaired endocytic pathway and proplatelet formation in Dnm2-null MKs. (A) Representative clathrin heavy chain, EEA1, APPL1, and calreticulin immunofluorescence images of Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrow MKs. Bars represent 10 µm. (B) Dnm2fl/fl (n = 10) and Dnm2fl/flPf4-Cre (n = 16) fetal liver–derived MKs extending proplatelets were counted 18 hours after BSA gradient (day 4 of culture) and expressed as percentage of total MKs (mean ± SEM; ***P < .0001). Representative light microscopy images of fetal liver–derived Dnm2fl/fl (C) and Dnm2fl/flPf4-Cre (D) MK-forming proplatelets in vitro. Areas within boxes in panels C1 and D1 are shown at higher magnification in panels C2 and D2, respectively. Bars represent 50 µm. Representative α-tubulin immunofluorescence images of fetal liver–derived Dnm2fl/fl (E) and Dnm2fl/flPf4-Cre (F) MK-forming proplatelets in vitro. Bars represent 20 µm.
Impaired proplatelet formation of Dnm2-null fetal liver–derived MKs
To further study platelet biogenesis, we capitalized on an in vitro MK differentiation system in which fetal liver cells were isolated and homogenized at embryonic day 14.5 and cultured in the presence of TPO for 4 days.25 Only 18.8 ± 1.8% of Dnm2-null MKs (mean ± SEM; n = 16) produced proplatelets compared with 36.2 ± 1.4% of control MKs (mean ± SEM; n = 10; P < .0001) (Figure 3B). Dnm2-null proplatelets were less complex in their structure, frequently shorter in length, curled, and less branched (Figure 3D, F) compared with controls (Figure 3C, E).
Impaired Mpl endocytosis in Dnm2-null platelets
Because Mpl surface expression is regulated by clathrin-dependent endocytosis,37-40 we investigated the role of DNM2 in platelet Mpl-mediated endocytosis. Dnm2-null platelets expressed Mpl normally, as indicated by immunoblot analysis (Figure 4A). In control platelets, Mpl surface expression was maximally decreased to 54.4 ± 5.7% (mean ± SEM; n = 5) of resting values after 10 minutes of incubation with 50 ng/mL of TPO, as seen by flow cytometry analysis (Figure 4B). In contrast, Mpl expression was only decreased to 93.4 ± 1.2% (mean ± SEM; n = 5; P < .0001) of resting values in Dnm2-null platelets at the same time point, indicating a defect in Mpl endocytosis in the absence of DNM2.
Impaired Mpl endocytosis in Dnm2-null platelets. (A) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelet lysates corresponding to 2 µg of protein were subjected to SDS-PAGE and probed for Mpl and actin as indicated. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelets were incubated with 50 ng/mL of TPO for 0 to 20 minutes at 37°C, incubated with a rabbit antibody directed against the extracellular domain of Mpl or control rabbit IgG, incubated with Alexa Fluor 488-labeled goat anti-rabbit IgG antibody, and analyzed by flow cytometry. Data represent anti-Mpl minus control IgG mean fluorescence intensities and are expressed as percentage of resting values (mean ± SEM; n = 4 in each group; **P < .01; ***P < .0001). (C) Plasma TPO levels in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 4 in each group; ***P = .0007). (D) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelets were incubated with 50 ng/mL of TPO for 0 to 20 minutes at 37°C, lysed, subjected to SDS-PAGE, and probed for phosphorylated JAK2 (pJAK2; Tyr1007/1008) and total JAK2, as indicated. (E) Densitometry analysis of the ratio of pJAK2 to total JAK2 (mean ± SEM; n = 4 in each group; *P < .05; **P < .01; ***P < .0001).
Impaired Mpl endocytosis in Dnm2-null platelets. (A) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelet lysates corresponding to 2 µg of protein were subjected to SDS-PAGE and probed for Mpl and actin as indicated. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelets were incubated with 50 ng/mL of TPO for 0 to 20 minutes at 37°C, incubated with a rabbit antibody directed against the extracellular domain of Mpl or control rabbit IgG, incubated with Alexa Fluor 488-labeled goat anti-rabbit IgG antibody, and analyzed by flow cytometry. Data represent anti-Mpl minus control IgG mean fluorescence intensities and are expressed as percentage of resting values (mean ± SEM; n = 4 in each group; **P < .01; ***P < .0001). (C) Plasma TPO levels in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 4 in each group; ***P = .0007). (D) Dnm2fl/fl and Dnm2fl/flPf4-Cre platelets were incubated with 50 ng/mL of TPO for 0 to 20 minutes at 37°C, lysed, subjected to SDS-PAGE, and probed for phosphorylated JAK2 (pJAK2; Tyr1007/1008) and total JAK2, as indicated. (E) Densitometry analysis of the ratio of pJAK2 to total JAK2 (mean ± SEM; n = 4 in each group; *P < .05; **P < .01; ***P < .0001).
Unrestrained Mpl signaling in Dnm2-null platelets
We further investigated the Mpl signaling cascade in Dnm2-null platelets, which requires JAK2 phosphorylation and activation.41 Incubation of control platelets with 50 ng/mL of TPO resulted in JAK2 tyrosine phosphorylation on activation loop Tyr1007/1008 that began at 2 minutes and became maximal at 5 minutes (Figure 4D-E), consistent with previous observations.42 Dnm2-null platelets had decreased expression of JAK2. Most of JAK2 was constitutively tyrosine phosphorylated in the absence of TPO, and phosphorylation was only slightly enhanced by incubation with TPO (Figure 4D-E). Taken together, our data show that the Mpl signaling cascade, particularly JAK2 phosphorylation, is constitutively active in Dnm2-null platelets due to impaired Mpl endocytosis.
Massive MK hyperplasia and MF in Dnm2fl/flPf4-Cre mice
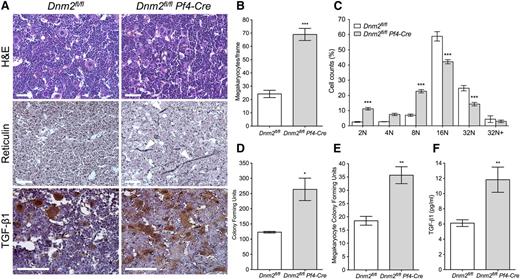
Bone marrow megakaryopoiesis was investigated in Dnm2fl/flPf4-Cre mice by H&E staining (Figure 5A, top row). Bone marrow sections of Dnm2fl/flPf4-Cre mice showed 69.0 ± 4.6 MKs per visual field compared with 24.2 ± 2.7 in Dnm2fl/fl animals (mean ± SEM; n = 5 in each group; P < .0001), a 2.9-fold increase (Figure 5B). Further examination of freshly isolated bone marrow MKs revealed a reduced ploidy in Dnm2fl/flPf4-Cre mice (Figure 5C), indicating more immature MKs in these animals, with 16N and 32N MKs representing 56.5 ± 1.4% of total bone marrow MKs (mean ± SEM; n = 5) compared with 83.7 ± 1.5% in Dnm2fl/fl mice (mean ± SEM; n = 3; P < .0001).
Bone marrow MK hyperplasia and MF in Dnm2fl/flPf4-Cre mice. (A) H&E, reticulin, and TGF-β1 staining of Dnm2fl/fl and Dnm2fl/flPf4-Cre femur bone marrow sections. Sections shown are representative of 5 mice for each genotype. Arrowheads in panel A (middle row) point to reticulin fibers. Bars represent 20 µm. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrow MK counts (mean ± SEM; n = 5 in each group; ***P < .0001). (C) Percentage of bone marrow Dnm2fl/fl and Dnm2fl/flPf4-Cre MK ploidy (mean ± SEM; n = 5 in each group; ***P < .001). (D) Bone marrow CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; *P = .0188). (E) Bone marrow MK CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; **P = .0034). (F) Bone marrow TGF-β1 levels in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; **P = .0044).
Bone marrow MK hyperplasia and MF in Dnm2fl/flPf4-Cre mice. (A) H&E, reticulin, and TGF-β1 staining of Dnm2fl/fl and Dnm2fl/flPf4-Cre femur bone marrow sections. Sections shown are representative of 5 mice for each genotype. Arrowheads in panel A (middle row) point to reticulin fibers. Bars represent 20 µm. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrow MK counts (mean ± SEM; n = 5 in each group; ***P < .0001). (C) Percentage of bone marrow Dnm2fl/fl and Dnm2fl/flPf4-Cre MK ploidy (mean ± SEM; n = 5 in each group; ***P < .001). (D) Bone marrow CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; *P = .0188). (E) Bone marrow MK CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; **P = .0034). (F) Bone marrow TGF-β1 levels in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; **P = .0044).
The number of multipotential hematopoietic progenitor CFUs was determined after isolation of bone marrow cells of Dnm2fl/flPf4-Cre mice and their culture in the presence of cytokines (Figure 5D). Dnm2fl/flPf4-Cre mice had 263.7 ± 36.9 bone marrow CFUs compared with 123.0 ± 2.6 in Dnm2fl/fl mice (mean ± SEM; n = 3 in each group; P = .0188). MK-specific CFUs were also significantly increased in Dnm2fl/flPf4-Cre bone marrow, with 35.7 ± 3.2 CFUs compared with 18.5 ± 1.7 in Dnm2fl/fl mice (mean ± SEM; n = 6 in each group; P = .0034) (Figure 5E). Thus, both multipotential hematopoietic progenitor and MK-specific CFUs were increased approximately twofold in Dnm2fl/flPf4-Cre bone marrow.
Dnm2fl/flPf4-Cre bone marrow showed a marked increase in reticulin fibers compared with that of control (Figure 5A, middle row, arrowheads), consistent with MF. Bone marrow levels of TGF-β1, a critical component of MF development,43 were significantly increased in Dnm2fl/flPf4-Cre mice, with 11.82 ± 1.66 pg/mL (mean ± SEM; n = 6) compared with 6.12 ± 0.44 pg/mL in Dnm2fl/fl mice (mean ± SEM; n = 7; P = .0044), an approximate twofold increase (Figure 5F). Histologic analysis of bone marrow showed association of TGF-β1 staining with MKs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (Figure 5A, bottom row).
Extramedullary hematopoiesis and splenomegaly in Dnm2fl/flPf4-Cre mice
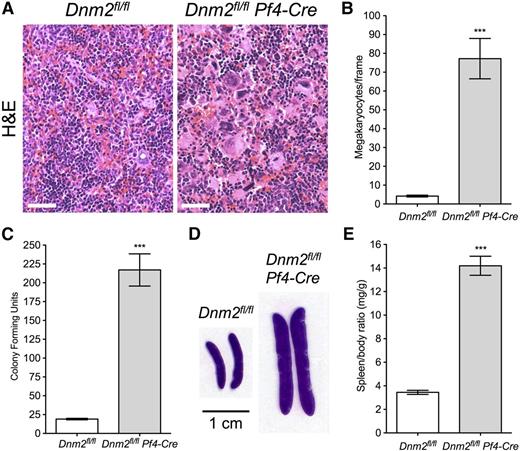
Spleen megakaryopoiesis was further investigated by H&E staining in Dnm2fl/flPf4-Cre mice (Figure 6A). Spleens of Dnm2fl/flPf4-Cre mice showed 77.2 ± 10.7 MKs per visual field compared with only 4.2 ± 0.5 in Dnm2fl/fl mice (mean ± SEM; n = 5 in each group; P < .0001), an ∼18-fold increase (Figure 6B). Strikingly, Dnm2fl/flPf4-Cre spleens had 217.0 ± 21.4 multipotential hematopoietic progenitor CFUs compared with only 19.0 ± 1.0 in control animals (mean ± SEM; n = 3 in each group; P = .0008), an ∼11-fold increase (Figure 6C), consistent with EMH.
Extramedullary hematopoiesis and splenomegaly in Dnm2fl/flPf4-Cre mice. (A) H&E staining of Dnm2fl/fl and Dnm2fl/flPf4-Cre spleens. Sections shown are representative of 5 mice for each genotype. Bars represent 20 µm. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre spleen MK counts (mean ± SEM; n = 5 in each group; ***P < .0001). (C) Spleen CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; ***P = .0008). (D) Dnm2fl/fl and Dnm2fl/flPf4-Cre spleen sections. Sections shown are representative of 15 mice for each genotype. (E) Spleen/body weight ratios of Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 15 in each group; ***P < .0001).
Extramedullary hematopoiesis and splenomegaly in Dnm2fl/flPf4-Cre mice. (A) H&E staining of Dnm2fl/fl and Dnm2fl/flPf4-Cre spleens. Sections shown are representative of 5 mice for each genotype. Bars represent 20 µm. (B) Dnm2fl/fl and Dnm2fl/flPf4-Cre spleen MK counts (mean ± SEM; n = 5 in each group; ***P < .0001). (C) Spleen CFUs in Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 6 in each group; ***P = .0008). (D) Dnm2fl/fl and Dnm2fl/flPf4-Cre spleen sections. Sections shown are representative of 15 mice for each genotype. (E) Spleen/body weight ratios of Dnm2fl/fl and Dnm2fl/flPf4-Cre mice (mean ± SEM; n = 15 in each group; ***P < .0001).
Consequently, Dnm2fl/flPf4-Cre mice rapidly developed severe splenomegaly (Figure 6D), which was observed at 3 weeks of age, our earliest experimental observations (data not shown). At 7 to 10 weeks of age, the spleen/body ratio of Dnm2fl/flPf4-Cre mice was 14.19 ± 0.81 mg/g compared with 3.45 ± 0.17 mg/g in Dnm2fl/fl mice (mean ± SEM; n = 15 in each group; P < .0001), a 4.1-fold increase (Figure 6E). Taken together, the data show that Dnm2fl/flPf4-Cre mice developed MF and consequent EMH and splenomegaly.
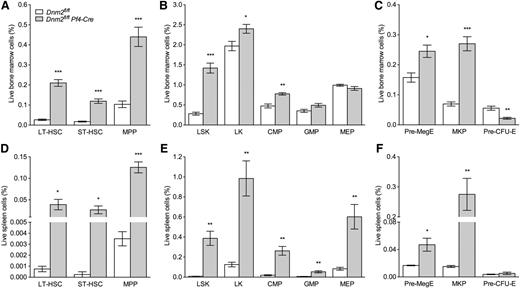
Increased HSPCs in Dnm2fl/flPf4-Cre mice
A quantitative assessment of the HSPC compartment of the bone marrow and spleen of Dnm2fl/fl and Dnm2fl/flPf4-Cre mice was performed using well-established immunophenotypic markers (Figure 7).32,33 Compared with Dnm2fl/fl mice, the lineagelowSca1+Kithigh (LSK) bone marrow compartment of Dnm2fl/flPf4-Cre mice was significantly expanded (fivefold), as was each of its subcompartments: CD150+CD48− LSK long-term hematopoietic stem cells (HSCs; 7.9-fold), CD150−CD48− LSK short-term HSCs (6.7-fold), and CD48+ LSK multipotent progenitors (4.2-fold) (Figure 7A-B). We also found a significant expansion in total myeloid progenitor lineagelowKithigh cells and in the common myeloid progenitor compartment of Dnm2fl/flPf4-Cre mice, in addition to a significant increase in Pre-MegE and MK precursor cells (3.9-fold) (Figure 7B-C). Pre-CFU-E cells were significantly reduced in the bone marrow of Dnm2fl/flPf4-Cre mice (2.6-fold), as compared with Dnm2fl/fl animals (Figure 7C).
Increased HSPCs in Dnm2fl/flPf4-Cre mice. Frequency of long-term (LT)-HSC, short-term (ST)-HSC, and multipotent progenitors (MPP) cells (A); LSK, lineagelowKithigh (LK), common myeloid progenitor (CMP), granulocyte/macrophage progenitor (GMP), and megakaryocyte/erythroid progenitor (MEP) cells (B); and Pre-MegE, MK precursor (MKP), and Pre-CFU-E cells (C) in Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrows. Frequency of LT-HSC, ST-HSC, and MPP cells (D); LSK, LK, CMP, GMP, and MEP cells (E); and Pre-MegE, MKP, and Pre-CFU-E cells (F) in Dnm2fl/fl and Dnm2fl/flPf4-Cre spleens. Data represent mean ± SEM (n = 4 in each group; *P < .05; **P < .01; ***P < .0001).
Increased HSPCs in Dnm2fl/flPf4-Cre mice. Frequency of long-term (LT)-HSC, short-term (ST)-HSC, and multipotent progenitors (MPP) cells (A); LSK, lineagelowKithigh (LK), common myeloid progenitor (CMP), granulocyte/macrophage progenitor (GMP), and megakaryocyte/erythroid progenitor (MEP) cells (B); and Pre-MegE, MK precursor (MKP), and Pre-CFU-E cells (C) in Dnm2fl/fl and Dnm2fl/flPf4-Cre bone marrows. Frequency of LT-HSC, ST-HSC, and MPP cells (D); LSK, LK, CMP, GMP, and MEP cells (E); and Pre-MegE, MKP, and Pre-CFU-E cells (F) in Dnm2fl/fl and Dnm2fl/flPf4-Cre spleens. Data represent mean ± SEM (n = 4 in each group; *P < .05; **P < .01; ***P < .0001).
We found similar findings in the splenic HSPC compartment of Dnm2fl/flPf4-Cre mice, in addition to a significant increase in granulocyte/macrophage progenitors and megakaryocyte/erythroid progenitors (Figure 7D-E). Pre-CFU-E cells were very rare, and unchanged in number, in spleens of Dnm2fl/flPf4-Cre mice, as compared with Dnm2fl/fl animals (Figure 7F). Taken together, our data indicate that the HSPC compartment of Dnm2fl/flPf4-Cre mice is expanded at each stage of differentiation, from primitive long-term HSCs through to committed MK precursor cells.
Discussion
Here we investigated the role of the large housekeeping GTPase DNM2 in mammalian MK maturation and platelet formation. Our data show that highly conserved DNM2-dependent endocytosis in MKs plays a previously unrecognized critical role in megakaryopoiesis, thrombopoiesis, and bone marrow homeostasis.
Earlier studies have suggested that DNMs, particularly DNM3, regulate megakaryopoiesis. DNM3 expression increases during maturation of cultured human MKs, and its knockdown by RNA interference inhibits MK growth and development.17,18 However, DNM3 appears to play a redundant role in platelet formation, because individuals who express in MKs a DNM3 truncate lacking the GTPase activity necessary for membrane fission have normal blood platelet counts and minimally affected mean platelet volumes.19
In contrast, MK lineage–specific DNM2 deletion demonstrates that DNM2 plays a critical role in MK maturation and platelet formation, because Dnm2fl/flPf4-Cre mice had severe macrothrombocytopenia. Bone marrow Dnm2-null MKs lacked a normal DMS, which appeared at times as a compact, narrow twisting membrane system of clathrin-coated vesicles, resembling the “clathrin-coated profiles” of Dnm1-null inhibitory synapses,12,13 and fetal liver–derived Dnm2-null MKs had impaired proplatelet formation in vitro. The endosome markers EEA1 and APPL1 were mislocalized in Dnm2-null MKs, consistent with severe defects in the endocytic pathway. In contrast, the endoplasmic reticulum chaperone calreticulin localized normally in Dnm2-null MKs, and Dnm2-null platelets expressed all major surface GPs, contained α-granules, and had moderately accelerated clearance in vivo. The results indicate that the exocytic pathway was affected to a lesser extent by DNM2 deletion in MKs. Thus, highly conserved DNM2-dependent endocytosis is an essential aspect of MK development and platelet production, particularly in the formation and organization of the MK DMS.
Our data are consistent with the observed association between germline DNM2 mutations K562E/del and thrombocytopenia in patients with dominant intermediate Charcot-Marie-Tooth disease (MIM:606482).20,21 Biochemically, mutations K562E/del are located in the pleckstrin homology domain “lipid binding pocket” and affect the ability of DNM2 to bind membrane phospholipids.44,45 Mutations K562E/del likely hinder the proper binding of DNM2 to MK intracellular membranes, resulting in impaired endocytosis and abnormal DMS formation.
Mpl endocytosis was severely impaired in Dnm2-null platelets, resulting in constitutive JAK2 phosphorylation and elevated circulating TPO levels. TPO is the main cytokine regulating megakaryopoiesis, and its receptor, Mpl, signals through JAK2 phosphorylation and activation, followed by phosphorylation and translocation to the nucleus of signal transducers and activators of transcription.41 Subsequently, Mpl is downregulated by clathrin-dependent endocytosis.37-40 Constitutive Mpl signaling in Dnm2-null platelets is consistent with unrestrained signaling due to impaired receptor-mediated endocytosis in cells lacking DNM2.9-11 Consistent with this, JAK2 expression was decreased in Dnm2-null platelets, likely reflecting preferential degradation of phosphorylated JAK2.46 Interestingly, somatic DNM2 loss-of-function mutations have been described in early T-cell precursor acute lymphoblastic leukemia.22,23 Based on the findings of our current study, we hypothesize that loss of DNM2 function in early T-cell precursor cells causes unrestrained signaling, growth, and proliferation in these cells, resulting in leukemogenesis. Additional studies in which DNM2 is deleted in HSCs are required to further evaluate this hypothesis.
Dnm2fl/flPf4-Cre mice developed HSPC expansion, MK hyperplasia, and MF, as indicated by bone marrow reticulin fibers, elevated bone marrow TGF-β1 levels, EMH, and splenomegaly. Emperipolesis, which is often observed in patients with MF,47 was also a common feature of Dnm2-null MKs. MF is a BCR-ABL1-negative myeloproliferative neoplasm, in which the clonal proliferation of malignant HSPCs results in the aberrant secretion of inflammatory cytokines and the development of bone marrow fibrosis.48,49 Approximately 50% of patients with MF carry the activating mutation V617F in JAK2, 5% carry activating mutations in Mpl, and 20% carry mutations in calreticulin.50-52
Recent studies confirmed the specificity of the Pf4-Cre model to mature MKs and platelets using Mpl and JAK2 deletion or JAK2-V617F expression.24,53-55 Accordingly, Dnm2fl/flPf4-Cre mice had normal erythrocyte counts and volumes, and granulocyte and monocyte counts were increased only by 30%, whereas these parameters are severely altered in JAK2-V617F knockin mouse models.56-60 DNM2 deletion in HSPCs is expected to severely alter erythropoiesis, because ubiquitous Dnm2 deletion is lethal in mice before embryonic day 8.5,8 a stage in which first erythrocytes circulate and the heterozygous DNM2 loss-of-function mutation V235G induced by chemical mutagenesis is associated with iron deficiency anemia due to impaired transferrin uptake in mice.61 Thus, abnormal Dnm2-null MKs and/or platelets are responsible for the severe bone marrow homeostasis defects observed in Dnm2fl/flPf4-Cre mice, including HSPC expansion and MF.
Mice with reduced platelet Mpl levels, either as a result of incomplete Mpl expression or due to JAK2-V617F expression, demonstrate HSPC expansion via elevated circulating TPO levels.62-65 Although decreased Mpl expression in JAK2-V617F knockin mice is likely due to increased Mpl-mediated endocytosis and subsequent degradation, MK-specific DNM2 deletion is expected to worsen the phenotype of these mice, because elevated circulating TPO levels would exacerbate their HSPC expansion and MK hyperplasia. Interestingly, specific JAK2-V617F expression in MKs using the Pf4-Cre model does not recapitulate the MF of Dnm2fl/flPf4-Cre mice or that of JAK2-V617F knockin mice.53 It is likely that JAK2-V617F expression is low in Jak2V617FPf4-Cre MKs and platelets compared with that of endogenous JAK2, making it poorly effective in inducing Mpl downmodulation.
Given that we observed an increase in both multipotential hematopoietic progenitor and MK-specific CFUs in vitro, it is likely that the HSPC expansion of Dnm2fl/flPf4-Cre mice is not only due to elevated TPO levels in vivo but also occurs as a result of enhanced responsiveness to TPO and/or other cytokines. DNM2-dependent endocytosis in MKs likely regulates other growth factors in addition to TPO. Notably, bone marrow TGF-β1 levels were elevated in Dnm2fl/flPf4-Cre mice. TGF-β1 levels are regulated by receptor-mediated endocytosis, and MKs express both TGF-β1 and its receptor.66,67 Further studies are required to determine whether TGF-β1, a critical component of MF development,43 or other growth factors are regulated in concert by DNM2-dependent endocytosis in MKs.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Wei Tong for the anti-Mpl antibody and Drs Antonija Jurak Begonja and John H. Hartwig for helpful discussions.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grants HL089224, HL107146, HL059561, HL104145, and HL109734; Canadian Institutes of Health Research grant MOP-119450; and Deutsche Forschungsgemeinschaft postdoctoral fellowship BE 5084/1-1 (M.B.).
Authorship
Contribution: All authors designed and performed the research, analyzed and interpreted the data, and contributed to the writing of the manuscript. H.F. designed the study and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Falet, Division of Hematology, Brigham and Women’s Hospital, One Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: hfalet@rics.bwh.harvard.edu.
References
Author notes
M.B. and S.G. contributed equally to this study.