Key Points
APC exhibits anticoagulant, antifibrinolytic, and antiinflammatory properties.
Intraperitoneal administration of APC effectively prevents postsurgical adhesion band formation.
Abstract
Postsurgical peritoneal adhesion bands are the most important causes of intestinal obstruction, pelvic pain, and female infertility. In this study, we used a mouse model of adhesion and compared the protective effect of activated protein C (APC) to that of the Food and Drug Administration–approved antiadhesion agent, sodium hyaluronate/carboxymethylcellulose (Seprafilm) by intraperitoneal administration of either APC or Seprafilm to experimental animals. Pathological adhesion bands were graded on day 7, and peritoneal fluid concentrations of tissue plasminogen activator (tPA), d-dimer, thrombin–antithrombin complex, and cytokines (IL-1β, IL-6, interferon-γ, tumor necrosis factor-α, transforming growth factor-β1) were evaluated. Inflammation scores were also measured based on histologic data obtained from peritoneal tissues. Relative to Seprafilm, intraperitoneal administration of human APC led to significantly higher reduction of postsurgical adhesion bands. Moreover, a markedly lower inflammation score was obtained in the adhesive tissues of the APC-treated group, which correlated with significantly reduced peritoneal concentrations of proinflammatory cytokines and an elevated tPA level. Further studies using variants of human APC with or without protease-activated receptor 1 (PAR1) signaling function and mutant mice deficient for either endothelial protein C receptor (EPCR) or PAR1 revealed that the EPCR-dependent signaling activity of APC is primarily responsible for its protective activity in this model. These results suggest APC has therapeutic potential for preventing postsurgical adhesion bands.
Introduction
Peritoneal adhesion bands are pathological fibrous tissues that join intraabdominal and intrapelvic organs to each other and to the abdominal wall. These bands develop in 67% to 93% of patients undergoing a general surgical operation and up to 97% of patients undergoing an open gynecological pelvic operation.1,2 Peritoneal adhesion bands are the most important causes of intestinal obstruction,3 pelvic pain,4 and female infertility.5 Many antiadhesive agents such as antiinflammatory drugs, antibiotics, fibrinolytic agents, and solid barriers have been used with limited success for the prevention of postsurgical peritoneal adhesion bands. Selected agents including sodium hyaluronate/carboxymethylcellulose (Seprafilm) and oxidized regenerated cellulose (Interceed) have been approved by the Food and Drug Administration (FDA) and are considered the gold standards for the prevention of postsurgical adhesion bands.6-10 These agents physically prevent adjacent tissues from contacting each other, thereby reducing the probability of adhesion band formation. A limitation of using these physical barriers is their site-specific nature, which requires the surgeon to predict where adhesions may occur in order to place these barriers accordingly.
Despite being a common sequel of wound healing process after abdominal surgery, the cellular and molecular mechanisms responsible for the formation of fibrous adhesion bands remain poorly understood. Injury to peritoneal mesothelial cells after surgery results in secretion of fibrin-rich exudates that constitute a suitable environment for migration and proliferation of underlying fibroblasts as a normal physiological response during the wound healing process.11-14 A delicate balance between fibrinolysis and cellular growth at this stage is essential to prevent the formation of adhesion bands. Impaired fibrinolysis can be associated with excess cellular growth, fibrosis, and deposition of collagen-rich extracellular matrix that leads to pathological adhesion bands. During peritoneal injury, mesothelial and fibroblast cells secret a significant amount of the profibrotic cytokine transforming growth factor (TGF)-β to the peritoneal cavity, thereby regulating different phases of the wound healing process (ie, inflammation, cellular migration, proliferation, angiogenesis, and tissue remodeling).11-14 It has been hypothesized that aberrant regulations of inflammation, fibrinolysis, and TGF-β signaling pathways make key contributions to the formation of postsurgical adhesion bands.14-16 In this study, we hypothesized that intraperitoneal administration of the anticoagulant and antiinflammatory protease activated protein C (APC) may inhibit postsurgical adhesion band formation. APC is an approved drug for treating adults with severe sepsis,17 although it has been recently pulled off the market by the manufacturer (Eli Lilly) due to its apparent lack of beneficial effect in a follow-up clinical study.18 Nevertheless, numerous in vitro and in vivo studies have firmly established potent antiinflammatory, cytoprotective, and profibrinolytic properties for APC in various injury model systems.19-24 APC, upon interaction with a vascular cell surface receptor, termed endothelial protein C receptor (EPCR), activates the G-protein coupled receptor, protease-activated receptor 1 (PAR1), thereby eliciting protective antiinflammatory and cytoprotective signaling responses.20,25,26 We tested our hypothesis in a mouse model of abdominal surgery, whereby we directly administered recombinant APC or its different variants possessing only anticoagulant or antiinflammatory activity24,26 to the peritoneal organs of wild-type and mutant mice deficient in either EPCR or PAR127-29 prior to closing the abdominal incision. Analyses of these results suggest that APC exhibits a markedly higher efficacy in preventing postsurgical peritoneal adhesion band formation in comparison with the FDA-approved Seprafilm. We demonstrate that the EPCR-dependent signaling activity of APC is primarily responsible for its protective properties in this model. Thus, APC holds the potential to be therapeutically used as a novel antiadhesive drug during general surgery.
Materials and methods
Animals
C57BL/6 mice were purchased from Jackson Laboratory. Transgenic EPCRδ/δ and PAR1−/− male mice have been previously described.27-29 Animals were backcrossed onto an inbred C57BL/6 background. All experiments involving animals were performed according to the guidelines for Care and Use of Laboratory Animals approved by Saint Louis University.
Experimental design
Eight-week-old C57BL/6 mice, weighing 25 to 30 g, were used for surgery in this study as described.30 Animals were randomly divided into 7 groups of 10 mice per group: group 1 had control animals with surgical abrasion plus saline (1 mL) as vehicle; group 2 had surgical abrasion plus sodium hyaluronate/carboxymethylcellulose (Seprafilm, Genzyme Biosurgery Corporation, Cambridge, MA) as a gold standard; group 3 had surgical abrasion plus 50 µg/kg recombinant APC-wild type (APC-WT) (in 1 mL saline) administered intraperitoneally; group 4 had surgical abrasion plus 50 µg/kg APC-2Cys; group 5 had surgical abrasion plus 50 µg/kg APC-E170A; group 6 had surgical abrasion plus 50 µg/kg of APC-S195A; and group 7 had surgical abrasion plus therapeutic unfractionated heparin (100 U/mouse). The optimal concentration of APC was determined by evaluating the effect of increasing concentrations of APC (10, 25, 50, 100, 200 µg/kg) APC on adhesion band formation (n = 10 mice for each group). The effect of APC on adhesion bands was evaluated using EPCR-deficient and PAR1 knockout mice. In this case, after surgery, 3 groups of 10 mice/group were treated with vehicle, Seprafilm, and APC-WT (50 µg/kg) as described above. Based on the weights of mice (25-30 g), the doses of APC ranged from 1.25 to 1.50 µg per mouse in all experiments. The surgical treatment was performed under a general anesthesia inhalant containing ethoxyethane (ether) and intraperitoneal injection of 3 mg/kg ketamine hydrochloride as described.10 All animal cares and procedures were performed according to the guidelines approved by the ethical committee of Saint Louis University.
Surgical methods, grading adhesion bands, and statistical analysis
Surgical methods, grading adhesion bands, histologic evaluation, and statistical analysis are described in the supplemental Materials available on the Blood Web site.
Results
APC infusion prevents adhesion band formation
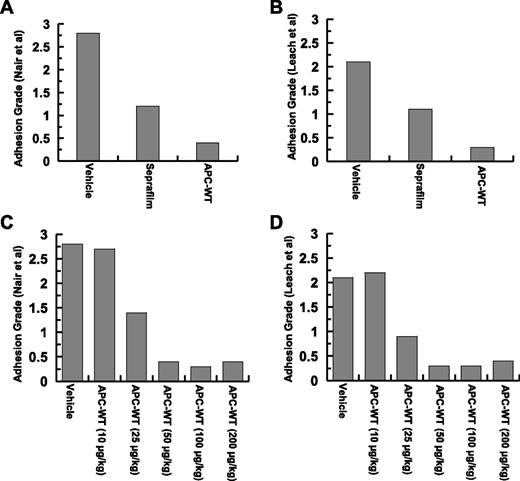
Grades of intraperitoneal adhesion bands (frequency and distribution) in experimental mice were measured 7 days postoperative according to 2 methods described by Nair et al.31 and Leach et al.6 (see supplementary Figure 1 and supplementary Table 1 for the grading systems). The average adhesion score frequency based on these systems for 10 mice per group is presented in Figure 1A-B. Analysis of data according to these 2 scoring systems suggests that APC inhibits adhesion bands to a significantly greater extent than Seprafilm. Indeed, no adhesion band was visible in mice treated with APC after 7 days. By contrast, most of adhesion bands remained unaffected in the Seprafilm-treated group over the 7-day observation period (Figure 1A-B). Furthermore, no mortality or complications (ie, bleeding) were observed in any of the experimental animals. The efficacy of several different concentrations of APC (10, 25, 50, 100, and 200 µg/kg) was evaluated and the APC concentration of 25 µg/kg was determined to be only slightly less active than the 50 µg/kg dose, which yielded the maximal protective effect in preventing adhesions. Increasing the concentration of APC to >50 µg/kg did not result in any additional protective effect (Figure 1C-D).
Analysis of postsurgical adhesion grades for Seprafilm- and APC-treated groups. (A) Adhesion grades for 10 mice (supplemental Table 1) based on Nair et al’s31 scale. (B) Adhesion grades for 10 mice (supplemental Table 1) based on Leach et al’s6 scale. Analysis of APC concentration dependence of adhesion grades based on Nair et al’s31 (C) and Leach et al’s6 (D) scoring systems.
Analysis of postsurgical adhesion grades for Seprafilm- and APC-treated groups. (A) Adhesion grades for 10 mice (supplemental Table 1) based on Nair et al’s31 scale. (B) Adhesion grades for 10 mice (supplemental Table 1) based on Leach et al’s6 scale. Analysis of APC concentration dependence of adhesion grades based on Nair et al’s31 (C) and Leach et al’s6 (D) scoring systems.
APC ameliorates peritoneal inflammation
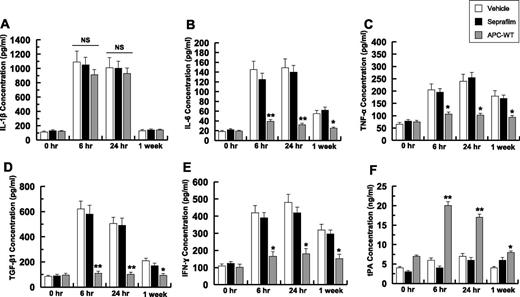
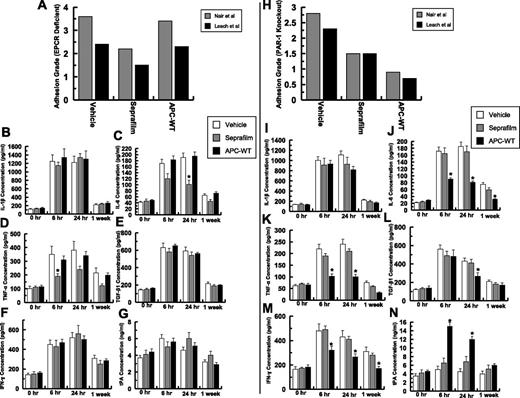
Peritoneal fluid concentrations of different cytokines were determined by enzyme-linked immunosorbent assay at 4 time points of 0 hours, 6 hours, 24 hours, and at 1-week postoperative (Figure 2). At the 6-hour time point, the IL-1 level was elevated greater than sixfold in the control and treatment groups and neither Seprafilm nor APC had statistically significant effects in the cytokine level (Figure 2A). The IL-1 level gradually declined and reached near the baseline level 1-week postoperative (Figure 2A). By contrast, the concentration of IL-6 was elevated in both control and Seprafilm-treated groups (Figure 2B). Although APC markedly inhibited the IL-6 level 6 hours postoperative, Seprafilm had minimal effect on the IL-6 level for the duration of the experiment (Figure 2B). Similarly, APC effectively inhibited tumor necrosis factor (TNF)-α, thus decreasing its secretion to a near baseline level at all 3 time points examined (Figure 2C). By contrast, the TNF-α level remained high in the Seprafilm group for 1-week postoperative (Figure 2C). Although the Seprafilm had no effect on the expression level of TGF-β, APC inhibited its secretion at both 6-hour and 24-hour time points (Figure 2D). APC also inhibited the expression of interferon (IFN)-γ, however, Seprafilm had no significant effect on the IFN-γ expression level (Figure 2E). The concentration of tissue plasminogen activator (tPA) in peritoneal fluid was markedly elevated in the APC group at 6 hours postoperative (Figure 2F), whereas Seprafilm had no effect on the tPA level for the duration of the experiment (Figure 2F). These results suggest that APC infusion, in contrast to Seprafilm, inhibited postsurgical proinflammatory responses associated with adhesion band formation.
Analysis of peritoneal fluid concentrations of cytokines and tPA. The concentrations of IL-1β (A), IL-6 (B), TNF-α (C), TGF-β1 (D), IFN-γ (E), and tPA (F) for all groups were measured by enzyme-linked immunosorbent assays. The data are shown as mean ± standard deviation (SD). *P < .05 and **P < .01 compared with saline-treated vehicle at each time point. NS, nonsignificant.
Analysis of peritoneal fluid concentrations of cytokines and tPA. The concentrations of IL-1β (A), IL-6 (B), TNF-α (C), TGF-β1 (D), IFN-γ (E), and tPA (F) for all groups were measured by enzyme-linked immunosorbent assays. The data are shown as mean ± standard deviation (SD). *P < .05 and **P < .01 compared with saline-treated vehicle at each time point. NS, nonsignificant.
Analysis of mRNA levels of proinflammatory cytokines
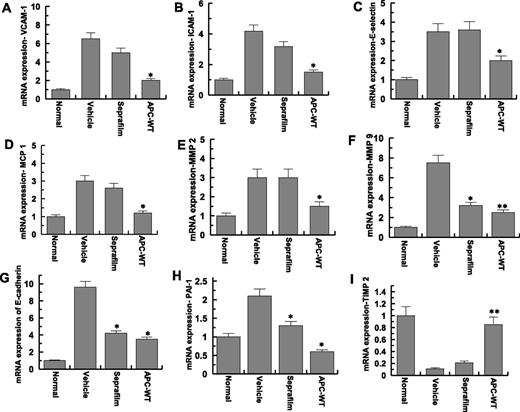
Adhesion bands and peritoneal tissues surrounding them for all mice were isolated one week post-surgery and the effect of APC and Seprafilm on the RNA levels of different cytokines were evaluated (Figure 3). As negative controls, a part of peritoneum from normal mice (no adhesion bands) in the mock surgery group was also removed and analyzed. Similar to saline treated group, the messenger RNA (mRNA) levels of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin were markedly elevated in the peritoneal tissues in the Seprafilm-treated group, however, APC effectively inhibited the expression levels of all 3 cell adhesion molecules (Figure 3A-C). Moreover, APC, but not Seprafilm, inhibited the mRNA levels of monocyte chemotactic protein-1 (Figure 3D) and matrix metalloproteinase-2 (Figure 3E). APC and to a lesser extent Seprafilm downregulated the mRNA levels of matrix metalloproteinase-9 (Figure 3F), E-cadherin (Figure 3G), and plasminogen activator inhibitor 1 (PAI-1) (Figure 3H), however, only APC could reverse injury-mediated downregulation of the mRNA level of the tissue inhibitor of metalloproteinase 2 (TIMP-2) (Figure 3I).
Analysis of mRNA expression of peritoneal tissues. Real-time quantitative polymerase chain reaction was used to analyze the mRNA expression levels in peritoneal adhesion bands of normal (no surgery), saline-treated (vehicle), Seprafilm-treated, and APC-WT-treated mice: (A), vascular cell adhesion molecule-1; (B), intercellular adhesion molecule-1; (C), E-selectin; (D), monocyte chemotactic protein-1; (E), matrix metalloproteinas-2; (F), matrix metalloproteinas-9; (G), E-cadherin; (H), PAI-1; (I), TIMP-2. ICAM-1, intercellular adhesion molecule-1; MCP 1, monocyte chemotactic protein-1; MMP 2, matrix metalloproteinase-2; MMP 9, matrix metalloproteinase-9; VCAM-1, vascular cell adhesion molecule-1. Data are shown as mean ± SD, n = 5. *P < .05 and **P < .01 compared with saline-treated vehicle.
Analysis of mRNA expression of peritoneal tissues. Real-time quantitative polymerase chain reaction was used to analyze the mRNA expression levels in peritoneal adhesion bands of normal (no surgery), saline-treated (vehicle), Seprafilm-treated, and APC-WT-treated mice: (A), vascular cell adhesion molecule-1; (B), intercellular adhesion molecule-1; (C), E-selectin; (D), monocyte chemotactic protein-1; (E), matrix metalloproteinas-2; (F), matrix metalloproteinas-9; (G), E-cadherin; (H), PAI-1; (I), TIMP-2. ICAM-1, intercellular adhesion molecule-1; MCP 1, monocyte chemotactic protein-1; MMP 2, matrix metalloproteinase-2; MMP 9, matrix metalloproteinase-9; VCAM-1, vascular cell adhesion molecule-1. Data are shown as mean ± SD, n = 5. *P < .05 and **P < .01 compared with saline-treated vehicle.
Adhesion band formation and postsurgical inflammation are inhibited by a signaling-selective APC variant
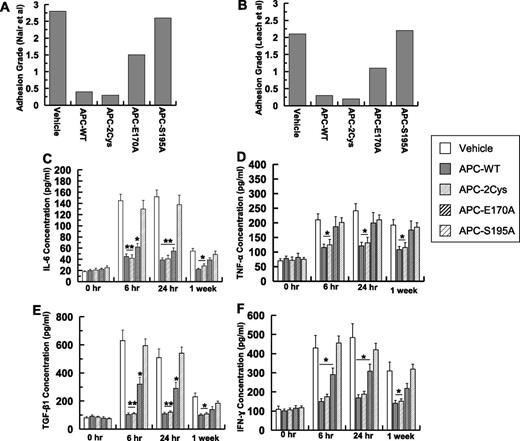
To understand the mechanism by which APC inhibits postsurgical adhesion band formation, grades of postsurgical intraperitoneal adhesions were measured in mice treated with 2 different APC variants, one that only has the signaling function (APC-2Cys) and the other that has normal anticoagulant but defective PAR1 signaling function (APC-E170A).24 Analysis of adhesion grades suggests that APC-2Cys (APC variant possessing only signaling function) inhibits adhesion band formation to a similar extent as APC-WT (Figure 4A-B; supplemental Table 1). Minimal adhesion bands were observed in mice treated with either APC-WT or APC-2Cys in a week-long study (Figure 4A-B). On the other hand, the APC-E170A derivative, which has normal EPCR binding and anticoagulant activity, but possesses no PAR1-dependent signaling function,24 exhibited significantly diminished protective activity (Figure 4A-B). The catalytically inactive APC-S195A mutant had no protective activity (Figure 4A-B), suggesting that the catalytic activity of APC is required for its protective function.
Analysis of adhesion grades and peritoneal fluid concentrations of cytokines for signaling-selective APC variants. (A) Adhesion grades for 10 mice (supplemental Table 1) based on Nair et al’s31 scale. (B) Adhesion grades for 10 mice (supplemental Table 1) based on Leach et al’s6 scale. The concentrations of IL-6 (C), TNF-α (D), TGF-β1 (E), IFN-γ (F) for all groups were measured by enzyme-linked immunosorbent assays. The data are shown as mean ± SD. *P < .05 and **P < .01 compared with saline-treated vehicle at each time point.
Analysis of adhesion grades and peritoneal fluid concentrations of cytokines for signaling-selective APC variants. (A) Adhesion grades for 10 mice (supplemental Table 1) based on Nair et al’s31 scale. (B) Adhesion grades for 10 mice (supplemental Table 1) based on Leach et al’s6 scale. The concentrations of IL-6 (C), TNF-α (D), TGF-β1 (E), IFN-γ (F) for all groups were measured by enzyme-linked immunosorbent assays. The data are shown as mean ± SD. *P < .05 and **P < .01 compared with saline-treated vehicle at each time point.
Similar to APC-WT, the signaling proficient APC-2Cys derivative effectively inhibited the IL-6 level 6-hour postoperative (Figure 4C). Interestingly, there was also a modest decrease in the IL-6 level in the APC-E170A group (Figure 4C). However, similar to Seprafilm group (Figure 2B), the cytokine level remained elevated with the APC-S195A group 1-week postoperative. Similar to APC-WT, APC-2Cys effectively inhibited TNF-α, thus decreasing its secretion to a near baseline level at all 3 time points examined (Figure 4D). By contrast, the TNF-α level remained high with both APC-E170A and APC-S195A groups for 1-week postoperative (Figure 4D). Similar to APC-WT, APC-2Cys dramatically inhibited the secretion of TGF-β at both 6-hour and 24-hour time points (Figure 4E). APC-E170A also exhibited a modest TGF-β inhibitory effect (6 hour and 24 hour), however, APC-S195A had no effect in preventing surgery-mediated peritoneal TGF-β expression (Figure 4E). APC-WT and APC-2Cys exhibited similar inhibitory activity profiles toward IFN-γ, thus reducing its expression level in peritoneal fluid (Figure 4F). The results suggest that the inhibition of adhesion bands and peritoneal inflammation require the signaling but not the anticoagulant activity of APC.
Both APC-WT and APC-2Cys effectively suppress inflammation scores in peritoneal tissues
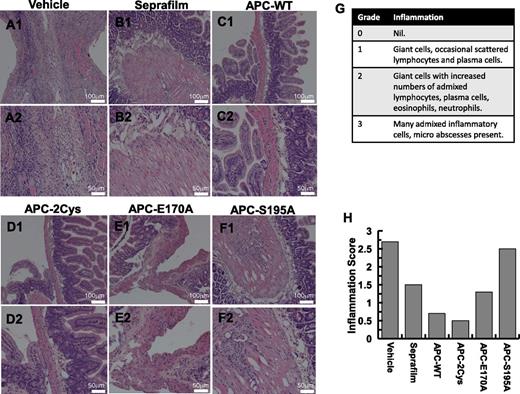
Hematoxylin and eosin staining was conducted to compare the infiltration of inflammatory cells to peritoneal tissues. The results in 2 magnifications for different groups: saline control (A), Seprafilm (B), APC-WT (C), APC-2Cys (D), APC-E170A (E), and APC-S195A (F) are presented in Figure 5. The inflammation scores, evaluated by 2 assessors in a double-blind study based on the histologic data are presented in Figure 5G-H. In the saline-treated control group, many inflammatory cells were recruited to peritoneal tissues and some microabscesses could be observed (Figure 5A). The same cell types, but to a lesser extent, could be detected in the photomicrographs of the Seprafilm group (Figure 5B). In contrast to both the control and Seprafilm groups, the number of inflammatory cells was markedly decreased and essentially no microabscesses could be observed in the APC-WT and APC-2Cys groups (Figure 5C-D). There were only a few scattered leukocytes in the APC-WT and APC-2Cys groups. Inflammation scores, as determined in a double-blind study on a scale of 0 to 3,10,32 are presented in Figure 5G. Statistically significant differences were observed between mice receiving Seprafilm, APC-WT, APC-2Cys, and APC-E170A derivatives from those receiving saline or APC-S195A. In comparison with the Seprafilm and APC-E170A groups, a significant decrease of the inflammation score was observed for APC-WT and APC-2Cys groups (Figure 5H). The total number of cells in the peritoneal cavity of experimental mice was determined to be 145 800/mL in untreated controls, 148 500/mL in the Seprafilm group, and 25 600 in the APC group, clearly showing the dramatic inhibitory effect of APC on migration of inflammatory cells to the peritoneal cavity in response to injury. The significantly lower inflammation scores of APC (also APC-2Cys) are consistent with their dramatic inhibitory effect on the expression levels of proinflammatory cytokines as demonstrated above in Figures 2 and 4.
Peritoneal tissue histology in 2 different magnifications. Representative images are derived from saline-treated control group (A1, A2), Seprafilm group (B1, B2), APC-WT group (C1, C2), APC-2Cys group (D1, D2), APC-E170A group (E1, E2), and APC-S195A (F1, F2). The numerical scoring of inflammation based on histology (G) is shown in panel (H).
Peritoneal tissue histology in 2 different magnifications. Representative images are derived from saline-treated control group (A1, A2), Seprafilm group (B1, B2), APC-WT group (C1, C2), APC-2Cys group (D1, D2), APC-E170A group (E1, E2), and APC-S195A (F1, F2). The numerical scoring of inflammation based on histology (G) is shown in panel (H).
Anticoagulant and direct profibrinolytic effects of APC do not contribute to the inhibition of adhesion band formation
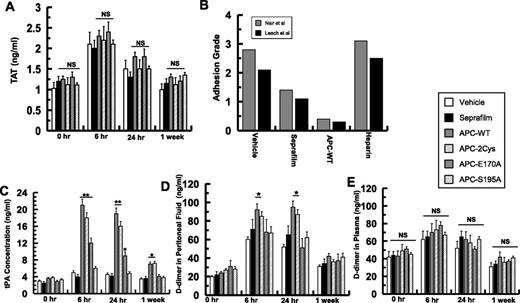
None of the APC variants exhibited a significant modulatory effect on the coagulation cascade (ie, thrombin generation) as judged from levels of thrombin-antithrombin (TAT) complex in plasma samples measured at different time points (Figure 6A). To further determine whether the inhibition of coagulation cascade makes any contribution to postsurgical adhesion band formation, the protective effect of therapeutic unfractionated heparin (100 U/mouse) was compared with the effects of Seprafilm and APC derivatives. Analysis of adhesion bands, according to the same scoring systems, revealed that heparin has no effect on the adhesion band formation (Figure 6B).
Analysis of coagulation and fibrinolytic markers in peritoneal fluids and plasma and the effect of heparin on adhesion band formation. (A) The concentrations of TAT complex for all groups were determined by an enzyme-linked immunosorbent assay. (B) The effect of therapeutic unfractionated heparin (100 U/mouse) on adhesion grades (10 mice/group) was compared with the effects of APC and Seprafilm using Nair et al’s31 and Leach et al’s6 scoring systems. (C) The concentrations of tPA in peritoneal fluids of different treatment groups were determined by an enzyme-linked immunosorbent assay. The concentrations of d-dimer in peritoneal fluids (D) or in plasma (E) for all groups were determined by an enzyme-linked immunosorbent assay. The data are shown as mean ± SD; NS, nonsignificant. *P < .05 and **P < .01 compared with saline-treated vehicle at each time point.
Analysis of coagulation and fibrinolytic markers in peritoneal fluids and plasma and the effect of heparin on adhesion band formation. (A) The concentrations of TAT complex for all groups were determined by an enzyme-linked immunosorbent assay. (B) The effect of therapeutic unfractionated heparin (100 U/mouse) on adhesion grades (10 mice/group) was compared with the effects of APC and Seprafilm using Nair et al’s31 and Leach et al’s6 scoring systems. (C) The concentrations of tPA in peritoneal fluids of different treatment groups were determined by an enzyme-linked immunosorbent assay. The concentrations of d-dimer in peritoneal fluids (D) or in plasma (E) for all groups were determined by an enzyme-linked immunosorbent assay. The data are shown as mean ± SD; NS, nonsignificant. *P < .05 and **P < .01 compared with saline-treated vehicle at each time point.
Interestingly, the concentration of tPA in peritoneal fluids was markedly elevated in both APC-WT and APC-2Cys groups 6 hours postoperative (Figure 6C). Similar to results presented above for cytokines, APC-E170A also modestly increased tPA secretion (Figure 6C). To further evaluate the effect of fibrinolysis on adhesion band formation, peritoneal and plasma d-dimer levels of experimental animals were measured at 3 different time points. d-dimer levels in peritoneal fluids were significantly elevated after 6 hours and 24 hours postoperative in both APC-WT and APC-2Cys groups (Figure 6D), but there were no differences in plasma d-dimer levels between different groups for the duration of the experiments (Figure 6E). To determine whether the profibrinolytic effect of APC, exerted via neutralization of PAI-1,21 contributed to its antiadhesive effect, the reactivity of APC derivatives with PAI-1 was analyzed. APC-WT and APC-E170A exhibited similar second-order rate constants of 1 × 103 M−1s−1, however, APC-2Cys exhibited ∼10-fold lower reactivity with PAI-1 (k2 = 1 × 102 M−1s−1), suggesting that direct interaction of APC with PAI-1 may not contribute to its protective function.
Effect of APC on adhesion bands in EPCR-deficient and PAR1 knockout mice
To determine whether the EPCR-dependent activation of PAR1 by APC is required for its protective activity, the same postsurgical adhesion experiments were conducted in genetically altered EPCR-deficient (EPCRδ/δ) mice, which express a greatly diminished amount of the receptor (<10% of cell-surface expression) and in PAR1−/− knockout mice. Analysis of adhesion grades according to Nair et al31 and Leach et al6 scoring systems indicated that EPCR is required for the protective activity of APC as evidenced by the APC-treated EPCRδ/δ mice exhibiting no improvement in their adhesion band scores (Figure 7A; supplemental Table 2). The same modest protective effect that was observed for Seprafilm in wild-type mice was also observed in EPCRδ/δ mice, supporting the reliability of the adhesion band scoring systems. By contrast to EPCRδ/δ mice, APC inhibited adhesion bands in PAR1−/− knockout mice, although the extent of the protective effect was rather lower than that observed in wild-type mice (Figure 7H; supplemental Table 3), suggesting that APC cleavage of PAR1 makes some contribution to its protective effect. However, the bulk of the protective effect of APC is mediated through EPCR-dependent but PAR1-independent mechanisms. Unlike in wild-type mice, APC had minimal effect on cytokines (Figure 7B-F) or tPA (Figure 7G) levels in EPCRδ/δ mice. Moreover, similar to adhesion grade scoring systems, APC exhibited an activity profile in PAR1−/− knockout mice that mirrored the wild-type mice, although the extent of the protective activity of APC in inhibiting proinflammatory cytokine secretion or enhancing tPA expression in PAR1−/− knockout mice was significantly decreased (Figure 7H-N).
Analysis of adhesion scores and peritoneal cytokine levels of EPCRδ/δ and PAR1−/− knockout mice. Adhesion scores for 10 EPCRδ/δ mice (supplemental Table 3) based on both Nair et al’s31 and Leach et al’s6 scales (A). The concentrations of IL-1β (B), IL-6 (C), TNF-α (D), TGF-β1 (E), IFN-γ (F), and tPA (G) for all EPCRδ/δ mice groups were measured by enzyme-linked immunosorbent assays. Adhesion scores for 10 PAR1−/− knockout mice (supplemental Table 4) based on both Nair et al’s31 and Leach et al’s6 scales (H). The concentrations of IL-1β (I), IL-6 (J), TNF-α (K), TGF-β1 (L), IFN-γ (M), and tPA (N) for all PAR1−/− knockout mice groups were measured by enzyme-linked immunosorbent assays. *P < .05 compared with saline-treated vehicle.
Analysis of adhesion scores and peritoneal cytokine levels of EPCRδ/δ and PAR1−/− knockout mice. Adhesion scores for 10 EPCRδ/δ mice (supplemental Table 3) based on both Nair et al’s31 and Leach et al’s6 scales (A). The concentrations of IL-1β (B), IL-6 (C), TNF-α (D), TGF-β1 (E), IFN-γ (F), and tPA (G) for all EPCRδ/δ mice groups were measured by enzyme-linked immunosorbent assays. Adhesion scores for 10 PAR1−/− knockout mice (supplemental Table 4) based on both Nair et al’s31 and Leach et al’s6 scales (H). The concentrations of IL-1β (I), IL-6 (J), TNF-α (K), TGF-β1 (L), IFN-γ (M), and tPA (N) for all PAR1−/− knockout mice groups were measured by enzyme-linked immunosorbent assays. *P < .05 compared with saline-treated vehicle.
Discussion
In this study, we have demonstrated that APC can effectively prevent postsurgical adhesion band formation in the peritoneal cavity of experimental animals. APC is an anticoagulant serine protease that downregulates the clotting cascade through the proteolytic degradation of factors Va and VIIIa.19-21 In addition to its anticoagulant activity, APC also possesses potent antiinflammatory and cytoprotective properties when it binds EPCR to activate PAR1, thereby inhibiting NF-κB and other proinflammatory signaling pathways.20,25,26 APC also prevents inflammation-mediated edema formation through the Rac1-dependent upregulation of the tight junction proteins that are responsible for maintaining the integrity of the vascular endothelium.20,25,26,33 In light of well-documented clinical findings that postsurgical adhesion bands are caused by injury-mediated inflammatory and wound healing processes,11-16 we reasoned that the intraperitoneal administration of APC after surgery may prevent adhesion bands through the inhibition of inflammatory responses. Thus, we compared the efficacy of APC to Seprafilm, as an FDA-approved gold standard in preventing postsurgical adhesion bands in our model system. In agreement with our hypothesis, we discovered that APC is markedly more effective than Seprafilm in preventing adhesion band formation after abdominal surgery. Unlike Seprafilm, which had a marginal effect on the expression profiles of cytokines, APC inhibited the secretion of IL-6, TNF-α, IFN-γ, and TGF-β by peritoneal tissues into the abdominal cavity. Interestingly, APC also markedly upregulated the expression of the fibrinolytic protease, tPA, in peritoneal tissues. These results are consistent with another study showing that recombinant APC can inhibit intraperitoneal adhesions in a rat model.34 However, this previous study did not provide any insight into the mechanisms through which APC inhibits peritoneal adhesions.
The observation that TGF-β was rapidly and highly induced after surgery suggests that TGF-β, through regulation of tissue remodeling, angiogenesis, and extracellular matrix deposition/turn over, plays a key (patho)physiological role during early hours of the surgery-mediated injury. As a fibrosis factor, TGF-β also plays an important role in regulating fibrinolysis and it is a potent inducer of PAI-1, which can inhibit fibrinolysis through inactivation of tPA.15 The activation of coagulation can also lead to fibrin deposition after injury and trauma mediated by the surgery. Although the natural fibrinolytic pathway can catalyze the clearance of fibrin, the unregulated inflammatory responses and signaling through TGF-β can result in excessive fibrin deposition and tissue fibrosis, thereby leading to formation of a pathological fibrin matrix on abdominal organs, tissues, and the lining of the peritoneal cavity. This can result in infiltration of fibroblasts and excessive deposition of collagen, thereby fortifying the matrix and facilitating the tight adherence of surrounding peritoneal tissues to each other and/or to the abdominal cavity. The most likely sources of TGF-β are peritoneal fibroblasts and activated macrophages, which can be recruited into the peritoneal cavity after surgery-mediated tissue injury. In support of this hypothesis, it has been shown that surgical-induced hypoxia can recruit macrophages to sites of injury and hypoxic treatments of peritoneal fibroblasts and macrophages can significantly increase expression levels of TGF-β by these cells.16 These surgical-induced inflammatory events are the primary cause of postsurgical adhesion bands and it was interesting to discover that all of these inflammatory responses were inhibited by APC as evidenced by the protease potently inhibiting expression of TGF-β and other cytokines during the early hours after the surgery. However, the inhibition of TGF-β by a specific monoclonal antibody did not result in complete inhibition of adhesion bands (data not shown), suggesting that the pathogenesis of the disease is multifactorial and that TGF-β signaling is only partially responsible for adhesion band formation.
The observation that APC induced the expression of tPA suggests that the protease can also upregulate fibrinolysis through enhancing plasminogen activation apart from its direct anticoagulant activity, which can inhibit fibrin deposition through inhibition of thrombin generation. In support of a protective role for enhanced fibrinolytic activity of APC, the concentration of d-dimer was significantly increased in the peritoneal fluids of APC-treated but not Seprafilm-treated groups. APC is also known to neutralize PAI-1,21 particularly when the inhibitor is bound to vitronectin,35 which is expected to be abundant in the extracellular matrix of abdominal tissues during the wound healing process. However, the direct neutralization of PAI-1 by APC did not appear to play a significant role in enhancing fibrinolysis because APC-2Cys, which inhibited adhesion bands similar to APC-WT, had a 10-fold lower reactivity with PAI-1, but APC-E170A, which had significantly diminished protective activity exhibited normal reactivity with PAI-1. Based on these data, we propose that APC-mediated downregulation of PAI-1 and TGF-β expression, together with the upregulation of tPA expression by peritoneal tissues, all mediated by the cellular signaling function of the protease, contributes to enhanced peritoneal fibrinolysis. It is worth noting that some protective role for tPA in inhibiting peritoneal adhesion formation has also been previously demonstrated.36
The results with the genetically altered mice, either deficient for EPCR or devoid of PAR1, together with those of APC derivatives suggest that the EPCR-dependent signaling function of APC is essential for the ability of APC to prevent postsurgical adhesion band formation. This is evidenced by the observation that APC-2Cys, which has dramatically decreased anticoagulant activity, exhibited a normal protective activity in both in vitro and in vivo assays; however, APC did not exhibit any activity in the same assays in EPCRδ/δ mice. Unlike interaction with EPCR, which was required for the protective function of APC, the activation of PAR1 by APC only contributed to the protective activity of the protease, but it was not strictly required for its antiadhesive function because APC significantly inhibited both cytokine generation and adhesion band formation in PAR1−/− knockout mice. A previous study, investigating the cytoprotective function of another nonanticoagulant APC in the LPS-induced mouse endotoxemia, which used the same EPCRδ/δ and PAR1−/− knockout mice, also noted that some EPCR-dependent beneficial effects of APC do not require PAR1.22 It is possible that in addition to PAR1, the EPCR-dependent activation of another receptor (in particular, PAR3) by APC is required for its full protective activity. In support of this hypothesis, it has been reported that the EPCR-dependent noncanonical cleavage of PAR3 at Arg-41 by APC also contributes to its cytoprotective function.37,38 Interestingly, APC-E170A, which has normal anticoagulant and EPCR-binding properties but cannot activate PAR1, exhibited partial protective activity in both inhibiting cytokine secretion and adhesion band formation. Further studies will be required to determine whether the partial protective activity of APC-E170A in wild-type mice and that of APC-WT in PAR1−/− knockout mice is due to activation of PAR3 by these proteases. The observations that the plasma TAT and d-dimer levels were essentially identical for all groups suggest that the direct anticoagulant activity of APC plays a minimal role in the cytoprotective function of the protease in this model. This is consistent with heparin not exhibiting any protective effect. Nevertheless, the catalytic activity of APC was required for its function because the APC-S195A mutant, which can also bind EPCR normally but has no catalytic activity, did not exhibit any protective property. Taken together, these results suggest that APC effectively prevents postsurgical adhesion band formation by enhancing fibrinolysis and inhibiting inflammatory mediators during the early days after the surgery through its EPCR-dependent signaling mechanism that is only partially dependent on PAR1 activation.
The results presented above strongly suggest that APC may be therapeutically developed as a safe and effective drug for preventing postoperative adhesion band formation in humans. APC has already been therapeutically used for treating adults with severe sepsis for more than a decade.17,18 The major drawback of APC was determined to be its enhancement of bleeding incidence in ∼2% of the treated patients.17 This effect of APC as a drug for preventing/treating postsurgical adhesion bands is not expected to be a factor because unlike with severe sepsis, in which APC is injected intravenously, the drug is administered intraperitoneally after surgery and a much lower dose of APC is required to effectively prevent adhesions. This proposal is supported by the observation that intraperitoneal administration of APC did not affect the coagulation cascade as determined by the analysis of the plasma TAT complex levels in all experimental animals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Audrey Rezaie for proofreading the manuscript.
This work was supported by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute (HL 101917 and HL 62565 to A.R.R.).
Authorship
Contribution: P.D. and S.M.H. designed and performed experiments; P.D. analyzed data; H.W. provided key reagents; and A.R.R. supervised the project and wrote the manuscript.
Conflict-of-interest disclosure: P.D. and A.R.R. are coinventors on a patent application pertaining to the methods using APC for preventing adhesion. The remaining authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Department of Biochemistry and Molecular Biology, St. Louis University School of Medicine, 1100 S Grand Blvd, St. Louis, MO 63104; e-mail: rezaiear@slu.edu.
References
Author notes
P.D. and S.M.H. contributed equally to this study.