Key Points
GRFS is a new composite end point useful for comparing HCT techniques and represents ideal post-HCT recovery.
In our cohort of 907 allogeneic HCT recipients, 1-year GRFS was 31%, with best outcomes in recipients of marrow from matched sibling donors.
Abstract
The success of allogeneic hematopoietic cell transplantation (HCT) is typically assessed as individual complications, including graft-versus-host disease (GVHD), relapse, or death, yet no one factor can completely characterize cure without ongoing morbidity. We examined a novel composite end point of GVHD-free/relapse-free survival (GRFS) in which events include grade 3-4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death in the first post-HCT year. In 907 consecutive University of Minnesota allogeneic HCT recipients (2000-2012), 1-year GRFS was 31% (95% confidence interval [CI] 28-34). Regression analyses showed age, disease risk, and donor type significantly influencing GRFS. Adults age 21+ had 2-fold worse GRFS vs children; GRFS did not differ beyond age 21. Adjusted for conditioning intensity, stem cell source, disease risk, age, and transplant year, HLA-matched sibling donor marrow resulted in the best GRFS (51%, 95% CI 46-66), whereas HLA-matched sibling donor peripheral blood stem cells were significantly worse (25%, 95% CI 20-30, P = .01). GRFS after umbilical cord blood transplants and marrow from matched unrelated donors were similar (31%, 95% CI 27-35 and 32%, 95% CI 22-42, respectively). Because GRFS measures freedom from ongoing morbidity and represents ideal HCT recovery, GRFS has value as a novel end point for benchmarking new therapies.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) applied for the cure of hematologic malignancies is associated with 2 main risk factors for poor outcomes: (1) transplantation-related morbidity/mortality and (2) mortality from disease relapse (relapse-related mortality). Previous efforts to mitigate 1 cause of mortality have often compromised the other. For example, efforts to reduce graft-versus-host disease (GVHD) risk by T-cell depletion of the allograft can lower transplantation-related morbidity/mortality, but can also increase relapse risk.1,2 Similarly, efforts at reducing relapse-related mortality with intensified chemotherapy or higher doses of radiation can lead to excess deaths from organ damage, infections, or GVHD.3 Therefore, clinical interventions cannot be assessed fully by focusing on either transplantation-related morbidity/mortality or relapse-related mortality, and neither reflects continuing but nonlethal morbidity.
Composite end points acknowledge that both survival and rates of other critical events are important when testing new therapies.4 Recently, the Blood and Marrow Transplant Clinical Trials Network recognized the potential utility of a composite end point in trials of allogeneic HCT; the novel composite end point of GVHD-free, relapse-free survival (GRFS) after HCT, defined as grade 3-4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death. Each of these GRFS components is clinically meaningful. GRFS therefore represents ideal recovery from HCT (at 1 year) and a measure of cure without ongoing morbidity. Using a cohort of 628 adult patients treated with tacrolimus (Tac) and methotrexate (MTX) as GVHD prophylaxis between 2006 and 2009, data from the Center for International Blood and Marrow Transplant Research determined that the 1-year probability of GRFS was 23% (95% confidence interval [CI] 20-26).5 Thus, only approximately one-quarter of adult patients transplanted for malignant disease survived without at least 1 of these major complications during the first 12 months after HCT. To better understand which factors influence GRFS for both pediatric and adult patients, we studied the individual events that compose GRFS and determined the overall GRFS in a large cohort of patients treated with uniform supportive care at our institution. In doing so, we sought to identify modifiable characteristics that could help optimize patients’ outcomes.
Methods
Study design
The objective of this retrospective, single-institution study was to assess the clinical benefit of allogeneic HCT using a novel composite end point of 1-year GRFS and to determine any clinical factors predictive of 1-year GRFS. GRFS events were defined as grade 3-4 acute GVHD, chronic GVHD requiring systemic immunosuppressive treatment, disease relapse, or death from any cause during the first 12 months after allogeneic HCT. The study sample included 907 consecutive pediatric and adult allogeneic HCT recipients from the University of Minnesota who underwent HCT for malignant disease between 2000 and 2012. Only first allogeneic HCT procedures were included in this analysis. Eligible patients included recipients of grafts from either an 8/8 allele HLA-matched sibling (with matching considered at HLA-A, HLA-B, HLA-C, HLA-DRB1), 8/8 HLA-matched unrelated donor (URD), single umbilical cord blood (UCB), or double UCB donors (with matching considered at HLA-A, HLA-B, and HLA-DRB1 for UCB grafts). Patients were excluded if they were recipients of grafts from HLA-mismatched siblings or HLA-mismatched URD because of their relative infrequency in our population and their known higher rates of GVHD. Haploidentical donor HCTs (n = 10) were also excluded because of their infrequency. Patients were also excluded if they received both peripheral blood stem cell (PBSC) plus marrow grafts to allow for donor source analyses (n = 9) or were treated with cyclosporine (Csa) and prednisone for GVHD prophylaxis (n = 44) because this GVHD prophylaxis regimen was used only briefly at our institution.
Patient and treatment characteristics
Clinical factors examined included year of transplant (2000-2007 or 2008-2012, a natural data defined cut point as a continuous variable and by quintiles), prior autologous transplant, age (<21 vs 21+, the natural cut point in the data), gender, diagnosis (acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome/myeloproliferative neoplasms/chronic myeloid leukemia, non-Hodgkin lymphoma/Hodgkin lymphoma/chronic lymphocytic leukemia, or other malignancy), cytomegalovirus serostatus, conditioning (myeloablative conditioning with total body irradiation, myeloablative conditioning without total body irradiation, reduced intensity conditioning (RIC, defined as regimens that incorporated low-dose total body irradiation at a dose of 200 cGy, busulfan-containing regimens with doses ≤8 mg/kg, or melphalan at doses ≤140 mg/m2) with anti-thymocyte globulin, or RIC without anti-thymocyte globulin, GVHD prophylaxis (Csa or Tac with MTX, Csa or Tac with mycophenolate mofetil, sirolimus, or T-cell depletion), donor type (matched sibling donor [MSD], matched URD, single UCB, or double UCB), stem cell source (marrow, PBSC, or cord blood), hematopoietic cell transplantation comorbidity index,6 and disease risk (standard or high risk). Disease risk was classified as standard or high risk based on the American Society for Blood and Marrow Transplantation Request for Information 2006 risk scoring schema (http://www.asbmt.org). Standard risk includes acute leukemias in first or second complete remission, chronic myeloid leukemia in first chronic phase, Hodgkin lymphoma or non-Hodgkin lymphoma in complete or partial chemotherapy-sensitive remission, and chronic lymphocytic leukemia in first remission. All other diseases were defined as high risk. Disease-free survival (DFS) was defined as the time from transplantation to relapse of the underlying malignancy for which the transplant was performed; overall survival (OS) was defined as the time from transplantation to death. All patients or their parents/guardians signed written informed consent allowing the use of their medical data in clinical research. All HCT and data collection protocols were reviewed and approved by the University of Minnesota Institutional Review Board.
Statistical analysis
Statistical comparisons across categorical factors, not including time-to-event data, were completed with the χ-square test. Continuous factors were compared by the Kruskal-Wallis test for nonparametric data. Kaplan-Meier estimates were used to determine the unadjusted probability of GRFS through 1-year posttransplant,7 with differences between the curves determined using log-rank tests. Cox regression was used to examine the independent effect of clinical factors on GRFS.8 Martingale residuals were used to test against nonproportionality.9 Double UCB grafts suggested a slight violation of the proportional hazards assumption, but because the curves did not cross, this factor was included in the final model owing to its importance. Final models, which were simultaneously checked stratifying by donor type, yielded similar results. The adjusted overall GFRS curves for various parameters were computed as average estimates of the pooled sample, weighted by the proportions of the significant variables in the regression models.10,11 The effect of confounding by year of transplant was further evaluated by the use of a frailty model, treating year of transplant as a random effect rather than a fixed effect that needed estimating.12,13 All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 907 HCT recipients were included in this study (Table 1). Of these, 558 received HCT from 2000 to 2007 and 349 received HCT during 2008-2012. The latter cohort had significantly more patients >40 years of age (52% vs 61%, P = .02). Accordingly, the use of RIC and GVHD prophylaxis with a calcineurin inhibitor plus mycophenolate mofetil was also more frequent in the latter cohort (both P < .001). Eight patients in this cohort received T-cell depletion as GVHD prophylaxis, and 13 patients received sirolimus-based GVHD prophylaxis. Otherwise, the vast majority of the patients received a standard calcineurin inhibitor-based GVHD prophylaxis regimen. More double UCB grafts were included in the later cohort as well (47% vs 38%, P = .009). Otherwise, demographic factors were similar in the 2 cohorts. Adults composed the majority of patients in each group stratified by donor type (86%), except single UCB recipients, in which the majority were <21 years old (62%).
Patient and treatment characteristics
| Variable . | Strata . | Study group . |
|---|---|---|
| N | 907 | |
| Age | <21 | 220 (24.3%) |
| ≥21 | 687 (75.7%) | |
| Age (y) | Median (range) | 43.8 (1-75) |
| Patient gender | Male | 536 (59.1%) |
| Female | 371 (40.9%) | |
| Gender match | Match | 366 (40.4%) |
| Mismatch | 541 (59.6%) | |
| Diagnosis | ALL | 219 (24.1%) |
| AML | 356 (39.3%) | |
| MDS/MPN/CML | 181 (20.0%) | |
| NHL/Hodgkin/CLL | 106 (11.7%) | |
| Other malignancy | 45 (5.0%) | |
| Diagnosis risk | High risk | 317 (35.0%) |
| Prior autologous HCT | Yes | 53 (5.8%) |
| CMV serostatus | Positive | 519 (57.2%) |
| Conditioning | MA | 494 (54.5%) |
| RIC | 413 (45.5%) | |
| GVHD prophylaxis | Csa or Tac with MTX | 263 (29.0%) |
| Csa or Tac with MMF | 623 (68.7%) | |
| Other | 21 (2.3%) | |
| Donor type | Matched sibling | 322 (35.5%) |
| Matched URD | 73 (8.0%) | |
| Single UCB | 135 (14.9%) | |
| Double UCB | 377 (41.6%) | |
| Stem cell source | Marrow MSD | 53 (5.8%) |
| PBSC MSD | 269 (29.7%) | |
| Marrow URD | 62 (6.8%) | |
| PBSC URD | 11 (1.2%) | |
| UCB | 512 (56.4%) | |
| Year of HCT | 2000-2007 | 558 (61.5%) |
| 2008-2012 | 349 (38.5%) |
| Variable . | Strata . | Study group . |
|---|---|---|
| N | 907 | |
| Age | <21 | 220 (24.3%) |
| ≥21 | 687 (75.7%) | |
| Age (y) | Median (range) | 43.8 (1-75) |
| Patient gender | Male | 536 (59.1%) |
| Female | 371 (40.9%) | |
| Gender match | Match | 366 (40.4%) |
| Mismatch | 541 (59.6%) | |
| Diagnosis | ALL | 219 (24.1%) |
| AML | 356 (39.3%) | |
| MDS/MPN/CML | 181 (20.0%) | |
| NHL/Hodgkin/CLL | 106 (11.7%) | |
| Other malignancy | 45 (5.0%) | |
| Diagnosis risk | High risk | 317 (35.0%) |
| Prior autologous HCT | Yes | 53 (5.8%) |
| CMV serostatus | Positive | 519 (57.2%) |
| Conditioning | MA | 494 (54.5%) |
| RIC | 413 (45.5%) | |
| GVHD prophylaxis | Csa or Tac with MTX | 263 (29.0%) |
| Csa or Tac with MMF | 623 (68.7%) | |
| Other | 21 (2.3%) | |
| Donor type | Matched sibling | 322 (35.5%) |
| Matched URD | 73 (8.0%) | |
| Single UCB | 135 (14.9%) | |
| Double UCB | 377 (41.6%) | |
| Stem cell source | Marrow MSD | 53 (5.8%) |
| PBSC MSD | 269 (29.7%) | |
| Marrow URD | 62 (6.8%) | |
| PBSC URD | 11 (1.2%) | |
| UCB | 512 (56.4%) | |
| Year of HCT | 2000-2007 | 558 (61.5%) |
| 2008-2012 | 349 (38.5%) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chromic myeloid leukemia; CMV, cytomegalovirus; MA, myeloablative conditioning; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma.
Impact of clinical factors on GRFS
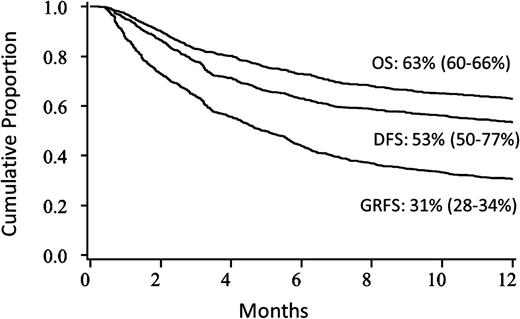
For the entire cohort, the unadjusted Kaplan-Meier estimate of 1-year GRFS was 31% (95% CI 28-34). This compared with a 1-year DFS of 53% (95% CI 50-77) and OS of 63% (95% CI 60-66) in the cohort (Figure 1).
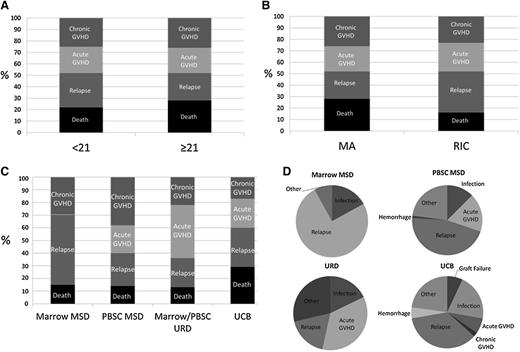
In comparing the GRFS events (defined as the first event) between donor types, relapse accounted for a greater proportion of the GRFS events in the pediatric group, whereas death without relapse or GVHD accounted for more of the GRFS events in adults (P < .01, Figure 2A). Relapse also accounted for a greater proportion of GRFS events in the RIC group (P < .01, Figure 2B). In comparing GRFS events between donor types (Figure 2C), MSD marrow was notable for no GRFS-defining acute GVHD events, whereas chronic GVHD accounted for the largest proportion of GRFS events in recipients of PBSC from MSD. The largest proportion of GRFS-defining events was attributed to acute GVHD in recipients of grafts from URD. Finally, death without relapse or GVHD was higher in recipients of UCB than other graft types
Distribution of individual components of GRFS. (A) Age (P < .01), (B) conditioning regimen (P < .01), (C) stem cell/donor type (P < .01), and (D) cause of death by donor type. MA, myeloablative conditioning.
Distribution of individual components of GRFS. (A) Age (P < .01), (B) conditioning regimen (P < .01), (C) stem cell/donor type (P < .01), and (D) cause of death by donor type. MA, myeloablative conditioning.
Causes of death
Disease relapse accounted for 75% of the deaths within the MSD marrow cohort (Figure 2D). Causes of death including graft failure (7%) and hemorrhage (5%) within the UCB group was absent in the other donor types except for a single fatal hemorrhage event within the MSD PBSC group. There were no deaths from acute GVHD in the MSD marrow group, although acute GVHD accounted for 7% of deaths within the UCB group, 17% of deaths within MSD PBSC group, and 36% of deaths within the URD group. Deaths from infections did not significantly differ between donor types.
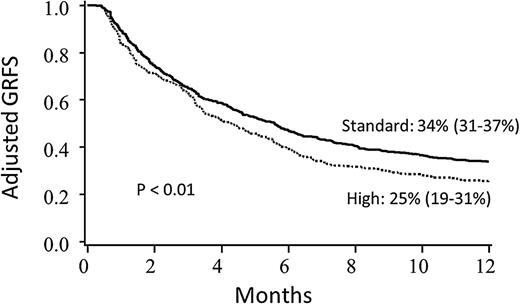
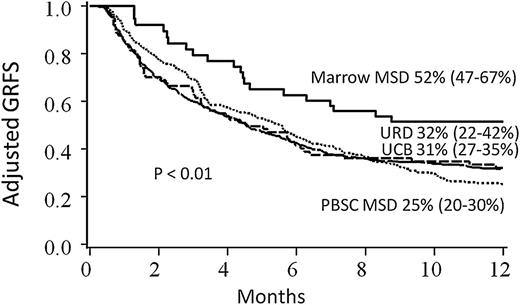
In regression analyses, conditioning, stem cell source, disease risk, and year of transplant significantly impacted GRFS, and thus Kaplan-Meier estimates of clinical factors on GRFS were adjusted for these variables (Table 2 for pediatrics; Table 3 for adults). Conditioning intensity did not impact GRFS after adjustment for other clinical variables for adult recipients; GRFS was significantly worse with reduced intensity conditioning for pediatric patients, although there were only 11 such patients. Adult patients with high-risk disease had significantly lower GRFS than patients with standard-risk disease (Figure 3). Notably, GRFS events in the high-risk disease cohort were not disproportionately from disease relapse; events were evenly divided among acute GVHD, chronic GVHD, relapse, and death (supplemental Table 1, available on the Blood Web site). In comparing donor types, MSD marrow grafts resulted in the best GRFS; GRFS after UCB HCT and matched URD were similar to each other, but worse than MSD marrow (Figure 4). When analyzing the impact of graft source on GRFS stratified by age group, marrow from MSD demonstrated the highest 1-year GRFS compared with other graft sources in both pediatric (67%, 95% CI 51-79) and adult (40%, 95% CI 12-67) cohorts, although differences between other graft types did not meet statistical significance (P = .06 for pediatrics and P = .57 for adults) when analyzed separately. GRFS was improved in the 2008-2012 cohort as compared with 2000-2007, although this was statistically significant for adults only.
Kaplan-Meier analysis of GRFS at 1 year and adjusted GRFS for the pediatric cohort (<21 years old)
| . | . | Frequency . | Survival estimate . | Log rank . | ||
|---|---|---|---|---|---|---|
| Factor . | Strata . | n . | Events . | Estimate . | CI 95% . | P value . |
| Total conditioning* | 220 | 105 | 52% | 45-59 | ||
| MA | 209 | 95 | 55% | 49-61 | <.01 | |
| RIC | 11 | 10 | 14% | 1-33 | ||
| Stem cell source* | Marrow-matched sibling | 43 | 14 | 66% | 52-80 | .16 |
| PBSC-matched sibling | 9 | 3 | 68% | 38-98 | ||
| Marrow-/PBSC-matched URD | 13 | 8 | 50 | 25-75 | ||
| Single UCB | 95 | 45 | 53% | 43-63 | ||
| Double UCB | 60 | 35 | 43% | 31-55 | ||
| Gender | Male | 138 | 67 | 51% | 43-59 | .73 |
| Female | 82 | 38 | 54% | 42-64 | ||
| Diagnosis | ALL | 110 | 51 | 54% | 44-62 | .77 |
| AML | 72 | 33 | 54% | 42-65 | ||
| MDS/MPN/CML | 22 | 12 | 45% | 24-64 | ||
| NHL/Hodgkin/CLL | 16 | 9 | 44% | 20-66 | ||
| Disease risk* | Standard risk | 178 | 80 | 54% | 47-61 | .54 |
| High risk | 42 | 25 | 45% | 31-76 | ||
| CMV serostatus | Negative | 91 | 38 | 58% | 47-68 | .17 |
| Positive | 129 | 67 | 48% | 39-56 | ||
| GVHD prophylaxis | Csa or Tac with MTX | 94 | 41 | 56% | 46-66 | .49 |
| Csa or Tac with MMF | 124 | 63 | 49% | 40-58 | ||
| Other | 2 | 1 | 50% | 1-91 | ||
| Year of HCT* | 2000-2007 | 136 | 71 | 47% | 39-55 | .09 |
| 2008-2012 | 84 | 34 | 61% | 51-71 | ||
| . | . | Frequency . | Survival estimate . | Log rank . | ||
|---|---|---|---|---|---|---|
| Factor . | Strata . | n . | Events . | Estimate . | CI 95% . | P value . |
| Total conditioning* | 220 | 105 | 52% | 45-59 | ||
| MA | 209 | 95 | 55% | 49-61 | <.01 | |
| RIC | 11 | 10 | 14% | 1-33 | ||
| Stem cell source* | Marrow-matched sibling | 43 | 14 | 66% | 52-80 | .16 |
| PBSC-matched sibling | 9 | 3 | 68% | 38-98 | ||
| Marrow-/PBSC-matched URD | 13 | 8 | 50 | 25-75 | ||
| Single UCB | 95 | 45 | 53% | 43-63 | ||
| Double UCB | 60 | 35 | 43% | 31-55 | ||
| Gender | Male | 138 | 67 | 51% | 43-59 | .73 |
| Female | 82 | 38 | 54% | 42-64 | ||
| Diagnosis | ALL | 110 | 51 | 54% | 44-62 | .77 |
| AML | 72 | 33 | 54% | 42-65 | ||
| MDS/MPN/CML | 22 | 12 | 45% | 24-64 | ||
| NHL/Hodgkin/CLL | 16 | 9 | 44% | 20-66 | ||
| Disease risk* | Standard risk | 178 | 80 | 54% | 47-61 | .54 |
| High risk | 42 | 25 | 45% | 31-76 | ||
| CMV serostatus | Negative | 91 | 38 | 58% | 47-68 | .17 |
| Positive | 129 | 67 | 48% | 39-56 | ||
| GVHD prophylaxis | Csa or Tac with MTX | 94 | 41 | 56% | 46-66 | .49 |
| Csa or Tac with MMF | 124 | 63 | 49% | 40-58 | ||
| Other | 2 | 1 | 50% | 1-91 | ||
| Year of HCT* | 2000-2007 | 136 | 71 | 47% | 39-55 | .09 |
| 2008-2012 | 84 | 34 | 61% | 51-71 | ||
Denotes adjusted variable.
Kaplan-Meier analysis of GRFS at 1 year and adjusted GRFS for adults (≥21 years old)
| . | . | Frequency . | Survival estimate . | Log rank . | ||
|---|---|---|---|---|---|---|
| Factor . | Strata . | n . | Events . | Estimate . | CI 95% . | P value . |
| Total | 687 | 525 | 24% | 20-27 | ||
| Conditioning* | MA | 285 | 229 | 21% | 17-25 | .29 |
| RIC | 402 | 296 | 26% | 22-30 | ||
| Stem cell source* | Marrow-matched sibling | 10 | 6 | 48% | 19-77 | .39 |
| PBSC-matched sibling | 260 | 214 | 19% | 15-23 | ||
| Marrow-/PBSC-matched URD | 60 | 45 | 28% | 17-39 | ||
| UCB (single) | 40 | 28 | 32% | 18-46 | ||
| UCB (double) | 317 | 232 | 25% | 21-29 | ||
| Gender | Male | 398 | 306 | 23% | 19-27 | .67 |
| Female | 289 | 219 | 24% | 19-29 | ||
| Diagnosis | ALL | 109 | 87 | 20% | 13-28 | .07 |
| AML | 284 | 206 | 27% | 22-33 | ||
| MDS/MPN/CML | 159 | 127 | 20% | 14-27 | ||
| NHL/Hodgkin/CLL | 90 | 65 | 28% | 19-37 | ||
| Other malignancies | 45 | 40 | 11% | 4-22 | ||
| Disease risk* | Standard risk | 412 | 302 | 27% | 23-31 | .02 |
| High risk | 275 | 223 | 19% | 15-24 | ||
| CMV serostatus | Negative | 297 | 221 | 26% | 21-31 | .22 |
| Positive | 390 | 304 | 22% | 18-26 | ||
| Prior auto-HCT | No | 639 | 492 | 23% | 20-26 | .27 |
| Yes | 48 | 33 | 31% | 19-44 | ||
| Year of HCT* | 2000-2007 | 422 | 342 | 20% | 16-24 | <.01 |
| 2008-2012 | 265 | 183 | 30% | 25-30 | ||
| . | . | Frequency . | Survival estimate . | Log rank . | ||
|---|---|---|---|---|---|---|
| Factor . | Strata . | n . | Events . | Estimate . | CI 95% . | P value . |
| Total | 687 | 525 | 24% | 20-27 | ||
| Conditioning* | MA | 285 | 229 | 21% | 17-25 | .29 |
| RIC | 402 | 296 | 26% | 22-30 | ||
| Stem cell source* | Marrow-matched sibling | 10 | 6 | 48% | 19-77 | .39 |
| PBSC-matched sibling | 260 | 214 | 19% | 15-23 | ||
| Marrow-/PBSC-matched URD | 60 | 45 | 28% | 17-39 | ||
| UCB (single) | 40 | 28 | 32% | 18-46 | ||
| UCB (double) | 317 | 232 | 25% | 21-29 | ||
| Gender | Male | 398 | 306 | 23% | 19-27 | .67 |
| Female | 289 | 219 | 24% | 19-29 | ||
| Diagnosis | ALL | 109 | 87 | 20% | 13-28 | .07 |
| AML | 284 | 206 | 27% | 22-33 | ||
| MDS/MPN/CML | 159 | 127 | 20% | 14-27 | ||
| NHL/Hodgkin/CLL | 90 | 65 | 28% | 19-37 | ||
| Other malignancies | 45 | 40 | 11% | 4-22 | ||
| Disease risk* | Standard risk | 412 | 302 | 27% | 23-31 | .02 |
| High risk | 275 | 223 | 19% | 15-24 | ||
| CMV serostatus | Negative | 297 | 221 | 26% | 21-31 | .22 |
| Positive | 390 | 304 | 22% | 18-26 | ||
| Prior auto-HCT | No | 639 | 492 | 23% | 20-26 | .27 |
| Yes | 48 | 33 | 31% | 19-44 | ||
| Year of HCT* | 2000-2007 | 422 | 342 | 20% | 16-24 | <.01 |
| 2008-2012 | 265 | 183 | 30% | 25-30 | ||
Denotes adjusted variable.
Adjusted Kaplan-Meier estimates of GRFS based upon disease risk. Estimates combine pediatric and adult patients and are adjusted for conditioning intensity, stem cell source, age, and year of transplant.
Adjusted Kaplan-Meier estimates of GRFS based upon disease risk. Estimates combine pediatric and adult patients and are adjusted for conditioning intensity, stem cell source, age, and year of transplant.
Kaplan-Meier estimate of GRFS. Estimates are based upon donor type, adjusted for conditioning intensity, stem cell source, disease risk, age, and year of transplant.
Kaplan-Meier estimate of GRFS. Estimates are based upon donor type, adjusted for conditioning intensity, stem cell source, disease risk, age, and year of transplant.
Multiple regression analyses
In multiple regression analysis (Table 4), factors that independently influenced GRFS were donor type, age, disease risk, and year of transplant. Pediatric recipients of marrow from MSD resulted in the most favorable outcomes and served as the reference group. Neither marrow from MSD in adult recipients (n = 10) nor PBSC from MSD for pediatric recipients (n = 9) differed significantly within the limitations of sample size. PBSC from MSD in adult HCT resulted in a nearly 4-fold worsening of GRFS (relative risk [RR] 3.6, 95% CI 2.1-6.4, P < .01) compared with pediatric recipients of MSD marrow. GRFS after URD HCT was significantly worse in adult recipients (RR 3.6, 95% CI 1.9-6.7). GRFS after UCB HCT was inferior to MSD marrow for both pediatric (RR 1.8, 95% CI 1.0-3.3) and adult (RR 3.6, 95% CI 2.0-6.3) recipients. Patients with high-risk malignancies had 30% worse GRFS compared with those with standard-risk disease (RR 1.3, 95% CI 1.0-1.5, P < .01). Year of HCT had a significant effect on GRFS, with a 20% improvement in 2008-2012 (RR 0.8, 95% CI 0.6-0.9). In a frailty model, we confirmed that donor type, age, and disease risk remained independent predictors of GRFS at 1 year, treating year of transplant as a random effect rather than fixed. Comorbidity as determined by the hematopoietic cell transplantation comorbidity index did not show an independent association with GRFS. In contrast to GRFS, in multivariate analysis, donor type did not impact DFS or OS. Variables that were independently associated with DFS were disease risk, with a 30% increase in relapse or death for high-risk disease (RR of relapse or death 1.3, 95% CI 1.0-1.6, P = .02) and earlier year of HCT (RR 0.7, 95% CI 0.5-0.8, P < .01 for 2008-2012 as compared with 2000-2007). For OS, high-risk disease (RR of death 1.3, 95% CI 1.0-1.6, P = .05) and year of HCT (RR 0.8, 95% CI 0.6-1.0, P = .04 for 2008-2012 compared with 2000-2007) were also independent risks in multivariate analysis, although grade 3-4 allogenic GVHD as a time-dependent covariate was also associated with poorer OS (RR 1.3, 95% CI 1.0-1.8, P = .05 for grade 3-4 allogenic GVHD vs grade 0-2).
Multiple regression analysis on risk of treatment failure as defined by GRFS (boldface denotes statistical significance
| Factors . | Strata . | n . | RR of GRFS (95% CI) . | P value . |
|---|---|---|---|---|
| Conditioning | MA | 494 | 1.0 | |
| RIC | 413 | 0.9 (0.8-1.1) | .48 | |
| Donor type | Marrow MSD (age <21) | 43 | 1.0 | Reference |
| Marrow MSD (age 21+) | 10 | 1.8 (0.2-3.5) | .73 | |
| PBSC MSD (age <21) | 9 | 1.0 (0.3-3.5) | .98 | |
| PBSC MSD (age 21+) | 260 | 3.6 (2.1-6.4) | <.01 | |
| Marrow/PBSC URD (age <21) | 13 | 1.9 (0.8-4.5) | .16 | |
| Marrow/PBSC URD (age 21+) | 60 | 3.6 (1.9-6.7) | <.01 | |
| UCB (age <21) | 155 | 1.8 (1.0-3.3) | .04 | |
| UCB (age 21+) | 357 | 3.6 (2.0-6.3) | <.01 | |
| Disease risk | Standard risk | 590 | 1.0 | |
| High risk | 317 | 1.3 (1.0-1.5) | <.01 | |
| Year of HCT | 2000-2007 | 558 | 1.0 | |
| 2008-2012 | 349 | 0.8 (0.6-0.9) | <.01 | |
| HCT-CI | Score of 0 | 235 | 1.0 | .23 |
| Score of 1-2 | 193 | 1.0 (0.8-1.3) | ||
| Score of 3+ | 267 | 1.2 (1.0-1.5) | ||
| Missing | 212 | 1.1 (0.9-1.5) |
| Factors . | Strata . | n . | RR of GRFS (95% CI) . | P value . |
|---|---|---|---|---|
| Conditioning | MA | 494 | 1.0 | |
| RIC | 413 | 0.9 (0.8-1.1) | .48 | |
| Donor type | Marrow MSD (age <21) | 43 | 1.0 | Reference |
| Marrow MSD (age 21+) | 10 | 1.8 (0.2-3.5) | .73 | |
| PBSC MSD (age <21) | 9 | 1.0 (0.3-3.5) | .98 | |
| PBSC MSD (age 21+) | 260 | 3.6 (2.1-6.4) | <.01 | |
| Marrow/PBSC URD (age <21) | 13 | 1.9 (0.8-4.5) | .16 | |
| Marrow/PBSC URD (age 21+) | 60 | 3.6 (1.9-6.7) | <.01 | |
| UCB (age <21) | 155 | 1.8 (1.0-3.3) | .04 | |
| UCB (age 21+) | 357 | 3.6 (2.0-6.3) | <.01 | |
| Disease risk | Standard risk | 590 | 1.0 | |
| High risk | 317 | 1.3 (1.0-1.5) | <.01 | |
| Year of HCT | 2000-2007 | 558 | 1.0 | |
| 2008-2012 | 349 | 0.8 (0.6-0.9) | <.01 | |
| HCT-CI | Score of 0 | 235 | 1.0 | .23 |
| Score of 1-2 | 193 | 1.0 (0.8-1.3) | ||
| Score of 3+ | 267 | 1.2 (1.0-1.5) | ||
| Missing | 212 | 1.1 (0.9-1.5) |
HCT-CI, hematopoietic cell transplant comorbidity index.
Discussion
We analyzed a novel composite end point, GRFS, which reflects the major complications of HCT. Using this new end point, only 31% of patients survived to 1 year without experiencing a GRFS-defining event. It is sobering to realize that although the majority of allogeneic HCT recipients survive 1 year (63% in this cohort), fewer than one-third of patients do so without a major complication. However, it is important to note that composite end points using a time-to-event analysis only measure the time to the first event and thus may not replace detailed analysis of the individual events that compose the end point. We used the Blood and Marrow Transplant Clinical Trials Network definition of GRFS. Although a composite end point is novel for a GVHD prophylaxis trial, the components that compose GRFS are all relevant to a successful HCT, particularly chronic GVHD requiring systemic therapy as an end point. Although difficult to fully characterize, chronic GVHD that results in impairment of performance status would also have merit as a component of a clinically meaningful composite end point.
Few factors that impact GRFS in our cohort were modifiable. Recipient age and disease risk depend on biologic factors, but also on the timing of referral. Advanced disease was associated with inferior GRFS. Donor type is primarily determined by the availability of a suitable HLA-matched sibling donor.14 In our center, GVHD prophylaxis regimens are linked to donor source and intensity of the conditioning regimen; thus, we could not assess an independent effect of GVHD prophylaxis regimen within this cohort.
In our study, pediatric recipients of marrow grafts from MSD demonstrated the best GRFS. Notably, PBSC, the most frequent stem cell source in adult HCT today,14 was associated with inferior GRFS. Increased chronic GVHD was observed in those receiving PBSC, consistent with prior studies.15-17 Although the frequent use of PBSC is unlikely to change in the foreseeable future, our results highlight the importance of reducing toxicities of HCT, especially acute and chronic GVHD, which disproportionately affected adult patients receiving PBSC grafts in our series. This challenge is already appreciated at transplant centers worldwide. Strategies such as T-cell depletion have been employed with the goal of reducing GVHD risk in such patients, but these strategies have tradeoffs, including increased infection and relapse risk, and thus significant improvements in survival have not yet been observed.2,18 A continuing Blood and Marrow Transplant Clinical Trials Network study (protocol 1203; https://web.emmes.com/study/bmt2/) is determining the impact of novel GVHD prophylaxis regimens on GRFS.5 Our study suggests that donor characteristics will have to be considered when analyzing the results of the Clinical Trials Network trial, although PBSC is the sole graft source allowed on the trial. Compared with PBSC, rates of chronic GVHD were lowest with recipients of UCB grafts, as previously described.19 In contrast, increased rates of death without GVHD or relapse have been described with UCB grafts, which is likely related to delayed engraftment compared with other graft sources.20,21 Because GRFS events vary by donor type, a study of personalized, risk-based GVHD prophylaxis, based upon donor, disease status, and other clinical factors, may optimize outcomes when all the GRFS components are considered. Importantly, this analysis does not suggest preferential use of UCB over URD for adults or pediatric patients, nor does it suggest preferential use of marrow over PBSC for MSD or URD HCT in adult recipients. Analysis of separate patient cohorts with increased representation of the different donor types and graft sources will be required for this purpose.
GRFS represents ideal recovery following HCT, without continuing morbidity, and differs significantly based upon patient age, disease risk, and donor type. It yields more information regarding complications of HCT than the simpler measurement of OS or DFS. Its use will better compare these clinically important outcomes that accompany disparate HCT techniques.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Michael Franklin for assistance in editing this manuscript.
This project was supported in part by the National Institutes of Health, National Cancer Institute (P30 CA77598 and P01CA111412) using the Biostatistics and Bioinformatics Shared Resource of the Masonic Cancer Center, University of Minnesota; and by grants from the National Cancer Institute (P01 CA65493) (B.R.B. and C.G.B.); the Children’s Cancer Research Fund (T.E.D., B.R.B., and M.L.M.); and Leukemia and Lymphoma Society Scholar in Clinical Research Award (R6029-07) (C.G.B.).
Authorship
Contribution: S.G.H., M.L.M., and D.J.W. conceived the study; T.E.D., S.G.H., M.L.M., and D.J.W. analyzed and interpreted data; S.G.H. wrote the manuscript; T.E.D., M.L.M., M.A., A.L., N.B., C.G.B., B.R.B., and D.J.W. edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel J. Weisdorf, Blood and Marrow Transplant Program, University of Minnesota, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: weisd001@umn.edu.