In this issue of Blood, Appelmann et al provide evidence for prolonged survival and prevention of resistance in a mouse model of Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) by combined targeting of the BCR-ABL kinase and Janus kinase 2 (JAK2) with dasatinib and ruxolitinib, respectively.1
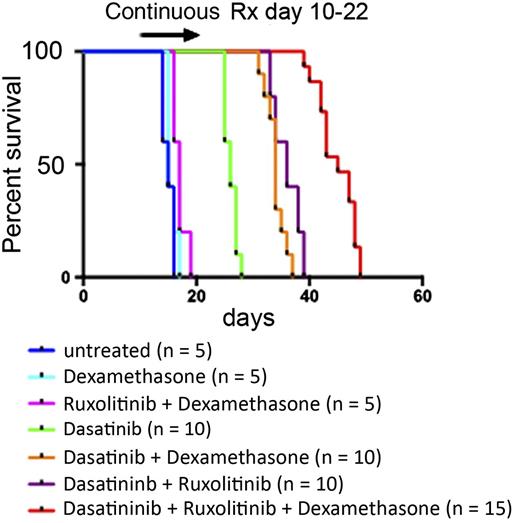
Shown is the survival of mice with Ph+ ALL that were treated with the indicated drug combinations for 13 days. See Figure 4B in the article by Appelmann et al that begins on page 1444.
Shown is the survival of mice with Ph+ ALL that were treated with the indicated drug combinations for 13 days. See Figure 4B in the article by Appelmann et al that begins on page 1444.
The Ph chromosome translocation results in the expression of the constitutively active BCR-ABL tyrosine kinase. BCR-ABL is the sole oncogenic driver in chronic myelogenous leukemia (CML) and, among other genetic aberrations, is also expressed in a subset of B-cell acute lymphoblastic leukemias (B-ALLs). Treatment of CML with the tyrosine kinase inhibitor imatinib or with one of its more potent successors, such as dasatinib, induces durable cytogenetic and molecular remissions so that a majority of CML patients can profit from a life expectancy that is similar to that of the normal population.2 In B-ALL, the presence of the Ph chromosome is among the worst prognostic factors. Responses of Ph+ ALL patients who are treated with BCR-ABL kinase inhibitors are short-lived, and relapse is frequently accompanied by the emergence of resistance-causing point mutations in the BCR-ABL kinase domain.3 Therefore, we must identify additional molecular targets that can complement chemotherapy and hematopoietic stem cell transplantation and improve patient survival. JAK tyrosine kinases, which are central components of cytokine and growth factor signaling pathways, may qualify as suitable targets in Ph+ ALL. JAK signaling supports the survival and proliferation of leukemia cells in the bone marrow microenvironment, and activated JAK kinases are driver oncogenes in many hematologic malignancies. Upon the identification of activating JAK2 mutations in myeloproliferative neoplasms, JAK2 kinase inhibitors were developed, of which the first-in-class drug ruxolitinib was approved for the treatment of myelofibrosis in 2011, and in late 2014 it was also approved for polycythemia vera.4 Of note, ruxolitinib improves disease-related symptoms, such as splenomegaly, and the quality of life of myelofibrosis patients, although paradoxically it has very little effect on the JAK2 mutant allele burden.
Appelmann et al1 have used a mouse model of Ph+ ALL to study the interplay of BCR-ABL and cytokine signaling to evaluate combined targeting of BCR-ABL and JAK2 on survival and relapse frequency. The authors used a well-characterized bone-marrow transplantation model that adequately recapitulates many aspects of human Ph+ ALL with its aggressive course, response to BCR-ABL inhibitors, frequent occurrence of relapse, and development of resistance by acquisition of kinase domain mutations. The characterization of leukemia-initiating B-cell progenitor cells in vitro showed a more than 20-fold decrease in dasatinib sensitivity upon cytokine stimulation. Inhibition of JAK2 with ruxolitinib restored dasatinib sensitivity and inhibition of activation of STAT3 and STAT5, 2 key transcription factors downstream of JAK2. In line with these results, genetic ablation of functional cytokine receptors resulted in prolonged survival of mice that were treated with dasatinib. These results formed the basis for targeting of cytokine signaling using ruxolitinib in combination with dasatinib.
Although ruxolitinib alone had no effect on leukemic cell burden or survival of mice, the combination with dasatinib increased its efficacy in further decreasing the number of leukemic cells and prolonging survival. Still, mice relapsed and died even under the dasatinib plus ruxolitinib combination regimen, for which involvement of the central nervous system and the inability of dasatinib to efficiently penetrate the blood-brain barrier could be responsible. On the basis of these observations, the authors then tested a triple combination with the corticosteroid dexamethasone, which is a core pillar of ALL treatment regimens and may penetrate to the brain. Strikingly, after a 7-day treatment period with the triple drug combination, the leukemic cell burden was found to be below the detection limit of the assay. After treatment discontinuation, survival of mice treated with the triple drug combination was prolonged compared with groups of mice treated with dasatinib plus dexamethasone and ruxolitinib plus dexamethasone, indicating that levels of residual leukemic cells that trigger relapse were strongly diminished with the triple combination (see figure). Furthermore, addition of ruxolitinib and dexamethasone reduced spleen weight and triggered long-term survival of some mice when they were continuously treated with the 3 drugs. Finally, the triple drug combination prevented the occurrence of dasatinib resistance. In contrast, point mutations in BCR-ABL were frequently detected at relapse in the groups of mice treated with dasatinib alone or with dasatinib plus ruxolitinib.
Combinatorial targeting of BCR-ABL and JAK2 has been discussed and debated passionately in the CML field.5 Conditional genetic knockout of JAK2 did not lead to differences in disease latency or disease phenotype in a bone marrow transplantation mouse model of CML similar to the one used by Appelmann et al. These data showed that JAK2 has no CML cell–intrinsic prosurvival role and argued against a possible benefit of inhibiting JAK2 with drugs.6 Conversely, JAK2 is an important player in providing the cytokine milieu in the hematopoietic stem cell niche for the creation of a sanctuary for leukemic stem cells to survive, self-renew, and differentiate. In addition, more primitive CML stem and/or progenitor cells may rely on cytokine-activated JAK2 signaling and therefore may be eliminated by ruxolitinib, whereas these cells are known to be able to survive independent of BCR-ABL signaling and therefore to be intrinsically resistant to BCR-ABL kinase inhibitors.7 Finally, recent evidence provided support for combined targeting of BCR-ABL and JAK2 by using a combination of the BCR-ABL inhibitor nilotinib with ruxolitinib that resulted in enhanced killing of primitive human CML stem and/or progenitor cells ex vivo with the expectation of limiting minimal residual disease burden in patients.8 In parallel, a phase 1/2 clinical trial was initiated that aims to identify the maximum tolerated dose of ruxolitinib that can be added to the standard of care treatment of CML patients with BCR-ABL tyrosine kinase inhibitors and also to determine whether levels of minimal residual disease can be decreased by the combination treatment.9 This trial will also provide valuable insight for a possible clinical evaluation of the combination of dasatinib, ruxolitinib, and dexamethasone in Ph+ ALL patients for which Appelmann et al provide a strong rationale.
Although the clinical success of BCR-ABL kinase inhibitors in CML and Ph+ ALL greatly diverged in the past, the addition of ruxolitinib, which in its primary disease indications shows little disease-modifying activity, may further improve outcome of CML patients and, on the basis of the promising data presented by Appelmann et al, may greatly improve Ph+ ALL therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.