Key Points
In a Ph+ ALL mouse model, dasatinib inhibition of the BCR-ABL kinase resensitizes residual leukemic B cells to Janus kinase inhibition.
Dasatinib, ruxolitinib, and dexamethasone together limit emergence of dasatinib-resistant BCR-ABL mutants and extend long-term survival.
Abstract
Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is initiated and driven by the oncogenic fusion protein BCR-ABL, a constitutively active tyrosine kinase. Despite major advances in the treatment of this highly aggressive disease with potent inhibitors of the BCR-ABL kinase such as dasatinib, patients in remission frequently relapse due to persistent minimal residual disease possibly supported, at least in part, by salutary cytokine-driven signaling within the hematopoietic microenvironment. Using a mouse model of Ph+ ALL that accurately mimics the genetics, clinical behavior, and therapeutic response of the human disease, we show that a combination of 2 agents approved by the US Food and Drug Administration (dasatinib and ruxolitinib, which inhibit BCR-ABL and Janus kinases, respectively), significantly extends survival by targeting parallel signaling pathways. Although the BCR-ABL kinase cancels the cytokine requirement of immature leukemic B cells, dasatinib therapy restores cytokine dependency and sensitizes leukemic cells to ruxolitinib. As predicted, ruxolitinib alone had no significant antileukemic effect in this model, but it prevented relapse when administered with dasatinib. The combination of dasatinib, ruxolitinib, and the corticosteroid dexamethasone yielded more durable remissions, in some cases after completion of therapy, avoiding the potential toxicity of other cytotoxic chemotherapeutic agents.
Introduction
The oncogenic fusion protein BCR-ABL, a constitutively active tyrosine kinase driven by a balanced translocation between chromosomes 9 and 22 (the Philadelphia chromosome, Ph), initiates both chronic myeloid leukemia (CML) and Ph-positive acute lymphoblastic leukemia (Ph+ ALL).1 De novo Ph+ ALL closely resembles the aggressive lymphoid blast crisis of CML and is prone to relapse even after combined treatment with potent second-generation inhibitors of the BCR-ABL kinase and intense chemotherapy.2-4 In addition to Ph, deletions of the CDKN2A/B (INK4A/B-ARF) tumor suppressor locus (encoding p16INK4a, p15INK4b, and p14ARF)5,6 and the IKFZ1 (IKAROS) gene7 (encoding a transcription factor required for lymphoid development)8 are frequently detected in de novo Ph+ ALL at diagnosis and in CML lymphoid, but not myeloid, blast crisis. These additional mutations contribute to aggressive Ph+ ALL by increasing the self-renewal of immature B cells, impairing their differentiation, and canceling BCR-ABL-induced apoptosis.5,6,9,10
Retroviral vector–mediated introduction of the p185BCR-ABL isoform into bone marrow (BM) progenitor cells derived from Arf-null C57BL/6 mice, followed by 7-day expansion of the transduced progeny under B-cell selective-culture conditions, yields polyclonal, cytokine-independent leukemia-initiating cells (LICs). Only 20 such LICs initiate ALL when infused into the tail veins of healthy, nonconditioned syngeneic mice and kill recipient animals within a month of inoculation.11 Therefore, BCR-ABL expression and Arf inactivation are sufficient to guarantee leukemogenesis in healthy recipient animals that initially retain normal hematopoietic and immune function. Leukemic mice respond very poorly to imatinib (Gleevec),10,11 but enter hematologic remission in response to treatment with much more potent second-generation tyrosine kinase inhibitors (TKIs) such as dasatinib (Sprycel).12 However, like human patients with Ph+ ALL,13 continuously treated animals ultimately relapse with the emergence of drug-resistant leukemic clones containing clinically relevant BCR-ABL mutations.12 Premature withdrawal of dasatinib when animals are in remission results in reemergence of leukemia, demonstrating that an occult reservoir of residual LICs has not been eliminated. However, unlike mice that relapse on continuous therapy, leukemic B cells from the latter animals lack BCR-ABL mutations and remain sensitive to TKIs ex vivo,10-12 suggesting that minimal residual disease (MRD) is dependent on salutary signaling within the hematopoietic microenvironment.
Cytokine signaling triggers Janus kinase (JAK)-mediated phosphorylation of signal transducers and activators of transcription (STATs) to induce expression of genes that support B-cell proliferation and survival.14 By directly stimulating STAT phosphorylation,15-17 BCR-ABL bypasses cytokine dependency18 and maintains JAK-independent expression of cyclin D2 and Mcl1, both of which are essential for LIC maintenance.10,19,20 Conversely, dasatinib-mediated inhibition of the BCR-ABL kinase might restore the requirement for cytokine-dependent JAK signaling and sensitize residual LICs to the JAK inhibitor ruxolitinib (Jakafi).21,22 We now report that the survival of dasatinib-treated mice with BCR-ABL-induced ALL is significantly extended in response to coadministration of ruxolitinib, even though ruxolitinib alone has no antileukemic activity. Addition of dexamethasone further reduced the leukemic burden, prevented central nervous system (CNS) relapse, and led to more prolonged survival, concordant with its established efficacy as a mainstay of multidrug regimens for ALL treatment.2-4,23 Prevention of relapse achieved by a nongenotoxic combination of targeted treatments provides a preclinical rationale for employing dasatinib, ruxolitinib, and dexamethasone in older Ph+ ALL patients who are ineligible for BM transplant or cannot tolerate cytotoxic chemotherapy.
Materials and methods
BM cell transduction, adoptive cell transfer, and leukemia development
Mice were housed at St. Jude Children’s Research Hospital and Memorial Sloan Kettering Cancer Center in facilities accredited by the American Association of Laboratory Animal Care. Procedures were performed in accordance with Institutional Animal Care and Use Committee and National Institutes of Health guidelines. BM cells from Arf−/− mice24 backcrossed onto a C57BL/6 background and expressing or lacking the interleukin (IL) receptor common γ chain11 (γC) were transduced with a replication-defective mouse stem cell virus coexpressing human BCR-ABL (p185) and luciferase (Luc2) and plated on autologous stroma for 7 days in IL-7 to select for lymphoid progenitor cells.10,25 On IL-7 withdrawal, polyclonal cytokine-independent BCR-ABL-positive LICs11 were expanded and cryopreserved in fetal bovine serum (FBS) and 10% dimethylsulfoxide. LICs were thawed and recovered in liquid culture for 3 days before intravenous injection into the tail veins of healthy, nonconditioned 8- to 10-week-old C57BL/6 mice (Jackson Laboratory). After intraperitoneal injection of d-luciferin (100 mg/kg; Caliper Life Sciences, Hopkinton, MA), leukemic infiltration was documented by bioluminescence as described previously12 using a Xenogen IVIS imager and Living Image software Version 4.3.1 (Caliper Life Sciences) for image processing. Fluctuations of signal intensities over a 3-log range correspond to the leukemic burden estimated by independent clinical criteria at necropsy.12
Response of cultured LICs to dasatinib and ruxolitinib
LICs (1 × 105/200 µL medium)11 were seeded in 96-well flat-bottom plates to which various concentrations of dasatinib were added with or without 10 ng/mL of IL-7 (R&D Systems) and 100 nM ruxolitinib. Three days later, overall cell viability relative to drug-free controls was determined using a CellTiter 96 AQueous assay kit (Promega). For measurement of STAT phosphorylation, LICs in 6-well culture dishes were treated for 8 hours with 1 nM dasatinib, 100 nM ruxolitinib, or both, in the presence or absence of IL-7. Proteins recovered from detergent-lysed cells and resolved on denaturing gels were transferred to polyvinylidene-difluoride membranes and immunoblotted10 with antibodies to phosphorylated and total STAT5 and STAT3. Antibodies were from Cell Signaling Technologies (C11C5, D3A7, 79D7) and Santa Cruz Biotechnologies (sc-835).
Assessment of leukemic burden by quantitative polymerase chain reaction
Leukemic cell infiltration at necropsy in BM and spleen was measured in genomic DNA using a TaqMan Fast Advanced Master Mix (Applied Biosystems) and an individually designed probe set to detect vector-encoded Luciferase-2 DNA (forward primer: TGAGCGGCTACGTTAACAAC, reverse primer: CACGATGAAGAAGTGCTCGT, and TaqMan probe: 6-FAM-CAGCCAGCCGTCCTTGTCGA-TAMRA; Applied Biosystems), as well as an internal control primer set (VIC-TAMRA) detecting the mouse Tfrc gene (4458367; Applied Biosystems). Standard curves were derived by mixing BCR-ABL (p185)-positive LICs with mixtures of nucleated BM cells or splenocytes at defined ratios; the reproducible detection limit is 1 LIC in 10 000 total cells (see supplemental Figure 1, available on the Blood Web site).
Preclinical therapeutics
Dasatinib (LC Laboratories, Woburn, MA) and ruxolitinib (Incyte, Wilmington, DE) were administered by oral gavage to recipient animals. Dasatinib dissolved in 80 mM citric acid (pH 3.1) was given at 10 mg/kg per dose.12,26 Ruxolitinib prepared in phosphate-buffered saline containing 0.1% Tween-20 was administered at 60 mg/kg per dose.21,22 Dexamethasone sodium phosphate solution (APP Pharmaceuticals, Schaumburg, IL) was added to drinking water at an initial concentration of 6 mg/L for the first week of treatment and at 3 mg/L thereafter.12 All drugs were administered starting 10 days after intravenous cell infusion when animals had developed a significant leukemic burden as documented by bioluminescence. Dosing schedules are indicated in the text and figure legends. Mice were observed daily; those that became moribund during trials (intense luciferase signals, dehydration, respiratory distress, hind limb paralysis, generalized seizures) had extensive leukemic involvement (elevated peripheral white blood cell counts, splenic and lymph node enlargement, leukemic BM and CNS infiltration seen histologically and confirmed by quantitative polymerase chain reaction [qPCR]) as documented previously.10-12 Kaplan-Meier curves were generated using GraphPad Prism (v.6.0c for Mac), and the log-rank Mantel-Cox test was applied for pairwise comparisons of survival data.
Analysis of BCR-ABL mutations
Mice injected with 2 × 105 LICs were allowed to develop disease for 10 days and then were left untreated (5 mice) or treated continuously with dasatinib, or dasatinib plus ruxolitinib, with or without dexamethasone for up to 180 days. Mice were euthanized when they showed overt signs of leukemic relapse. BM cells were harvested and genomic DNA was prepared from cells derived from 5 to 7 individual relapsed mice per treatment group. A 2-step nested PCR strategy was used to amplify the human ABL kinase domain (KD) from 200 ng of each genomic DNA by first amplifying the BCR-ABL junction (forward primer: CGTGGGCGTCCGCAAGACCCG; reverse primer: GCCAGGCTCTCGGGTGCAGTCC) and then using a different forward primer (GCGCAACAAGCCCACTGTCTATGG) to amplify just the ABL KD. Products were ligated into an EcoRV-restricted pBluescript II KS(−) plasmid, and 10 to 20 white colonies (selected on isopropyl β-D-1-thiogalactopyranoside) per DNA sample were picked and subjected to nucleotide sequencing.27,28 Mutations were called only when present in forward and reverse sequencing reactions and in more than 20% of clones.
Results
Abrogated cytokine signaling improves dasatinib response of BCR-ABL-positive LICs
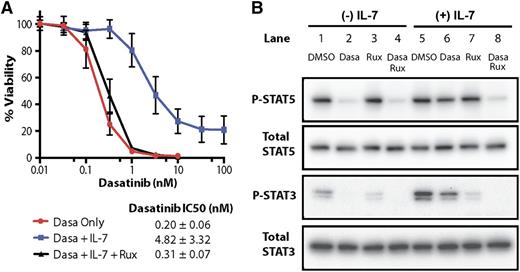
The BCR-ABL kinase circumvents cytokine receptor–mediated JAK signaling by phosphorylating specific STAT family members,15-17 reducing the dependency of cultured LICs on interleukins for their proliferation and survival.18 Nonetheless, BCR-ABL-positive LICs still respond to IL-7,10 which at saturating doses, lowered their sensitivity to dasatinib more than 20-fold (Figure 1A). Inhibition of STAT5 and STAT3 phosphorylation by dasatinib (Figure 1B, lane 2) was antagonized by IL-7 (lane 6). Although ruxolitinib treatment had no effect on STAT5 phosphorylation, it partially inhibited phosphorylation of STAT3 (lanes 3 and 7); however, concomitant inhibition of the BCR-ABL kinase with dasatinib resensitized IL-7-treated LICs to ruxolitinib (Figure 1A) and inhibited STAT phosphorylation (Figure 1B, lanes 4 and 8).
A blunted response of IL-7-treated LICs to dasatinib is reversed by ruxolitinib. (A) LICs were treated with the indicated concentrations of dasatinib, with or without 10 ng/mL of IL-7 and 100 nM ruxolitinib. Cell viability was assessed 72 hours later using a nonradioactive cell viability assay. The calculated inhibitory concentration required to arrest 50% of the cells (IC50) values and results shown are taken from 3 separate experiments, each yielding triplicate determinations for each data point. Notably, the IC50 of dasatinib is augmented ∼20-fold by IL-7 addition and is completely reversed by ruxolitinib. (B) Cells were cultured for 8 hours in the presence of vehicle (DMSO), 1 nM dasatinib, 100 nM ruxolitinib, or both drugs, either in the presence (+) or absence (−) of IL-7. Cells were lysed, and equal amounts of protein loaded per lane were resolved on denaturing gels and immunoblotted with antibodies to phosphorylated (P) and total STAT5 and STAT3. Dasa, dasatinib; DMSO, dimethylsulfoxide; Rux, ruxolitinib.
A blunted response of IL-7-treated LICs to dasatinib is reversed by ruxolitinib. (A) LICs were treated with the indicated concentrations of dasatinib, with or without 10 ng/mL of IL-7 and 100 nM ruxolitinib. Cell viability was assessed 72 hours later using a nonradioactive cell viability assay. The calculated inhibitory concentration required to arrest 50% of the cells (IC50) values and results shown are taken from 3 separate experiments, each yielding triplicate determinations for each data point. Notably, the IC50 of dasatinib is augmented ∼20-fold by IL-7 addition and is completely reversed by ruxolitinib. (B) Cells were cultured for 8 hours in the presence of vehicle (DMSO), 1 nM dasatinib, 100 nM ruxolitinib, or both drugs, either in the presence (+) or absence (−) of IL-7. Cells were lysed, and equal amounts of protein loaded per lane were resolved on denaturing gels and immunoblotted with antibodies to phosphorylated (P) and total STAT5 and STAT3. Dasa, dasatinib; DMSO, dimethylsulfoxide; Rux, ruxolitinib.
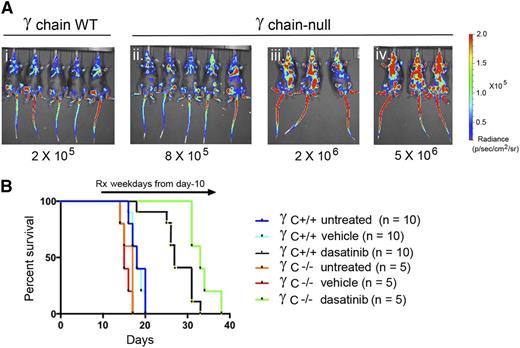
Consistent with the effects of IL-7 on cultured LICs, injected donor LICs lacking γC (required for IL-2, -4, -7, -9, -15, and -21 signaling) were less leukemogenic than their γC+/+ counterparts, and their serial dilution established that infusion of fourfold more γC−/− than γC+/+ LICs would generate an equivalent leukemia burden 10 days posttransplant (Figure 2Ai-ii). Additional groups of mice were infused with either 2 × 105 γC+/+ LICs or with a 10-fold greater number of γC−/− LICs (Figure 2Aiii), and dasatinib therapy was initiated in leukemic mice 10 days later. Although all untreated mice died within 3 weeks of receiving LICs, dasatinib treatment with a low-intensity regimen (10 mg/kg of drug once per day, 5 days per week) that is ineffective in maintaining remission12 led to survival benefits in both cohorts. Notably, even when challenged with 10-fold more γC−/− LICs, γC-null leukemias exhibited an improved response to dasatinib compared to γC+/+ leukemias (P = .014) (Figure 2B). Hence, interleukin signaling can facilitate leukemogenesis and insulate the response of LICs to targeted TKI therapy in vivo.
LICs lacking the cytokine receptor γC exhibit increased sensitivity to dasatinib therapy in vivo. (A) Mice infused with the indicated numbers of γC-positive LICs (i) or γC-null LICs (ii-iv) were imaged for vector luciferase activity 10 days later. Matched exposures document relative levels of fluorescence radiance indicated at the right. (B) Cohorts of mice receiving 2 × 105 γC -positive LICs (Ai) or 2 × 106 γC -null LICs (Aiii) were left untreated or were treated once daily 5 days per week with vehicle or dasatinib (10 mg/kg body weight) and euthanized when moribund. Despite their more significant leukemia burden at day 10 (Aiii vs Ai), mice receiving γC-null LICs exhibited a better response to dasatinib therapy. Statistics for pairwise comparisons: untreated vs vehicle (whether γC WT or null groups) are not significant; γC WT, vehicle vs dasatinib, P < .001; γC null, vehicle vs dasatinib, P < .001; dasatinib-treated γC WT vs dasatinib-treated γC null, P = .014. Rx, treatment; WT, wild-type.
LICs lacking the cytokine receptor γC exhibit increased sensitivity to dasatinib therapy in vivo. (A) Mice infused with the indicated numbers of γC-positive LICs (i) or γC-null LICs (ii-iv) were imaged for vector luciferase activity 10 days later. Matched exposures document relative levels of fluorescence radiance indicated at the right. (B) Cohorts of mice receiving 2 × 105 γC -positive LICs (Ai) or 2 × 106 γC -null LICs (Aiii) were left untreated or were treated once daily 5 days per week with vehicle or dasatinib (10 mg/kg body weight) and euthanized when moribund. Despite their more significant leukemia burden at day 10 (Aiii vs Ai), mice receiving γC-null LICs exhibited a better response to dasatinib therapy. Statistics for pairwise comparisons: untreated vs vehicle (whether γC WT or null groups) are not significant; γC WT, vehicle vs dasatinib, P < .001; γC null, vehicle vs dasatinib, P < .001; dasatinib-treated γC WT vs dasatinib-treated γC null, P = .014. Rx, treatment; WT, wild-type.
Effects of dasatinib, ruxolitinib, and dexamethasone during induction therapy
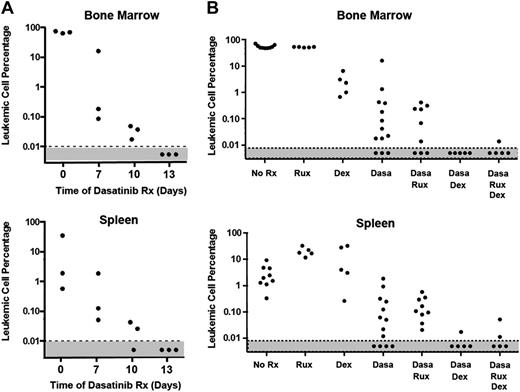
Ten days after infusion of 2 × 105 LICs, there is widely disseminated disease, including high peripheral white blood cell counts and significant leukemic infiltration into the BM, spleen, lymph nodes, and meninges.12 Bioluminescent imaging of living mice allows detection of leukemic cells over a 3-log range, whereas qPCR using TaqMan primers directed to the vector-containing Luc2 gene used for in vivo imaging can detect 1 LIC per 104 normal cells (supplemental Figure 1), enabling an independent and more sensitive measure of leukemic burden. Treatment of leukemic mice with twice-daily dasatinib (10 mg/kg) led to a drastic reduction in disease burden in both BM and spleen that fell below the limit of detection after 13 days (Figure 3A). Nonetheless, significant numbers of leukemia cells reemerged by 9 days after discontinuation of treatment, as indicated by the reappearance of intense whole-body bioluminescence signals in moribund mice that were roughly equivalent to those seen at start of therapy and by TaqMan qPCR analysis documenting 26% to 45% leukemic replacement in BM (data not shown). The posttherapeutic rebound reflects the rapid doubling time of residual leukemic cells, which leads to an ∼10-fold increase in leukemic burden every 3 days.11
Acute response of leukemic mice to drug treatment. (A) Dasatinib treatment rapidly reduces leukemia burden. Twelve mice injected intravenously with 2 × 105 LICs were allowed to develop leukemia over a 10-day period. Mice with equal leukemia burdens were randomized into groups of 3 mice, the first of which was left untreated and euthanized when moribund (time 0). Other groups were treated twice a day with dasatinib (10 mg/kg body weight) for 7, 10, or 13 days and euthanized immediately after the indicated times of treatment. Additional treated cohorts were allowed to undergo clinical relapse after dasatinib withdrawal at day 13 and were moribund by day 22 (see the “Effects of dasatinib, ruxolitinib, and dexamethasone during induction therapy” section). Bone marrow cells (top) and splenocytes (bottom) were harvested for TaqMan qPCR analysis performed with primers directed to the vector-containing Luc2 gene. The fraction of leukemic cells was interpolated from a standard curve created by mixing cultured BCR-ABL+, Arf−/− LICs with nucleated cells from BM or spleen at defined ratios (supplemental Figure 1). (B) Dexamethasone (Dex), but not ruxolitinib, reduces leukemic burden during 7-day induction therapy. Mice that received LICs and developed leukemia as in panel A were treated with the indicated drug combinations and euthanized. Because monotherapy with dasatinib for 13 days reduces the frequency of leukemic cells below the limit of detectability (panel A), a 7-day treatment period was chosen to allow any additional effects of ruxolitinib and dexamethasone to be recorded. Leukemia burden in BM (top) and spleen (bottom) was estimated by qPCR (TaqMan) as in panel A. Ruxolitinib treatment alone or in combination with dasatinib had no significant activity in acutely reducing the leukemic burden, whereas dexamethasone potentiated remission induction.
Acute response of leukemic mice to drug treatment. (A) Dasatinib treatment rapidly reduces leukemia burden. Twelve mice injected intravenously with 2 × 105 LICs were allowed to develop leukemia over a 10-day period. Mice with equal leukemia burdens were randomized into groups of 3 mice, the first of which was left untreated and euthanized when moribund (time 0). Other groups were treated twice a day with dasatinib (10 mg/kg body weight) for 7, 10, or 13 days and euthanized immediately after the indicated times of treatment. Additional treated cohorts were allowed to undergo clinical relapse after dasatinib withdrawal at day 13 and were moribund by day 22 (see the “Effects of dasatinib, ruxolitinib, and dexamethasone during induction therapy” section). Bone marrow cells (top) and splenocytes (bottom) were harvested for TaqMan qPCR analysis performed with primers directed to the vector-containing Luc2 gene. The fraction of leukemic cells was interpolated from a standard curve created by mixing cultured BCR-ABL+, Arf−/− LICs with nucleated cells from BM or spleen at defined ratios (supplemental Figure 1). (B) Dexamethasone (Dex), but not ruxolitinib, reduces leukemic burden during 7-day induction therapy. Mice that received LICs and developed leukemia as in panel A were treated with the indicated drug combinations and euthanized. Because monotherapy with dasatinib for 13 days reduces the frequency of leukemic cells below the limit of detectability (panel A), a 7-day treatment period was chosen to allow any additional effects of ruxolitinib and dexamethasone to be recorded. Leukemia burden in BM (top) and spleen (bottom) was estimated by qPCR (TaqMan) as in panel A. Ruxolitinib treatment alone or in combination with dasatinib had no significant activity in acutely reducing the leukemic burden, whereas dexamethasone potentiated remission induction.
Because 7-day dasatinib treatment is less effective in reducing the number of leukemic cells (Figure 3A), we reasoned that any additive therapeutic benefit of additional drugs could be determined at this time. Accordingly, we treated leukemic mice for 7 days with dasatinib (10 mg/kg twice daily), ruxolitinib (60 mg/kg once daily), and the corticosteroid dexamethasone, a mainstay of ALL treatment,23 either alone or in combination. Mice were euthanized after treatment, and TaqMan qPCR was performed to quantify MRD (Figure 3B). Dexamethasone alone, administered continuously in the drinking water, reduced the leukemic burden in BM and improved the response to dasatinib in lowering leukemic cell numbers in both BM and spleen. In marked contrast, ruxolitinib treatment had no immediate effect in reducing the leukemic burden during 7 days of induction therapy, either when administered alone or when combined with dasatinib. Moreover, further addition of ruxolitinib did not improve the response to dasatinib plus dexamethasone during the induction phase (Figure 3B).
Ruxolitinib and dexamethasone each extend survival of dasatinib-treated mice
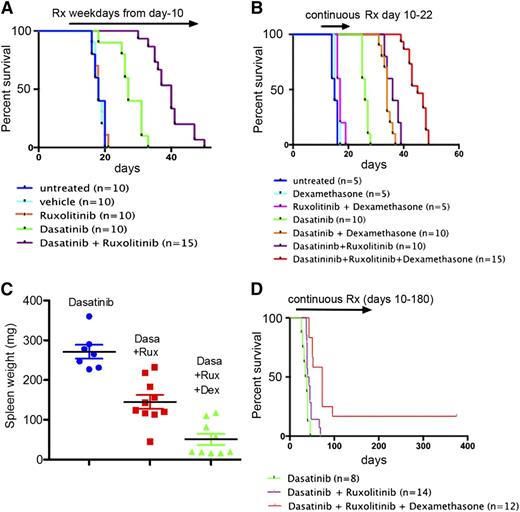
Because efficient inhibition of the BCR-ABL kinase by dasatinib allows reengagement of cytokine-mediated JAK-STAT signaling (Figure 1), we reasoned that any salutary effects of cytokines in maintaining MRD within the hematopoietic microenvironment after dasatinib treatment might well be overcome by ruxolitinib. To test this, leukemic mice that had received LICs were randomized after 10 days into different cohorts for treatment. Animals received either 10 mg/kg dasatinib, 60 mg/kg ruxolitinib, or both drugs combined once daily 5 days per week (weekdays only, as in Figure 2B). Animals were observed daily for signs of disease and imaged weekly, and relapses were documented at necropsy (see “Materials and methods”). As predicted, we did not observe a significant antileukemic effect with ruxolitinib alone (Figure 4A). However, overall survival was significantly improved in leukemic mice treated with the combination of both targeted therapies compared with dasatinib as a single agent (Figure 4A; P < .0001). Thus, although ruxolitinib had no effect in inducing leukemic remission, it had marked activity in improving progression-free survival.
Ruxolitinib and dexamethasone each prolong remission in dasatinib-treated mice. All mice were infused intravenously with 2 × 105 LICs. Mice that were left untreated or received drugs 10 days later were followed until they became moribund. (A) Mice treated for 5 days per week once per day by oral gavage with ruxolitinib alone (60 mg/kg body weight) exhibited no survival advantage compared with untreated or vehicle-treated mice, whereas mice receiving ruxolitinib plus dasatinib (10 mg/kg body weight) on the same dosing schedule showed a significant survival advantage over animals receiving dasatinib monotherapy (P < .001). (B) Mice were treated continuously for 13 days with the indicated drug combinations, after which therapy was discontinued. Dasatinib (10 mg/kg) was administered twice per day (unlike the once per day regimen in panel A) and ruxolitinib (60 mg/kg) administered once per day, each by oral gavage. Dexamethasone was added, as indicated, to the drinking water. The time to treatment failure (abscissa) reflects the level of MRD at the end of day 22. TaqMan PCR performed at necropsy on 4 animals that relapsed after combined treatment with dasatinib and ruxolitinib showed significant leukemic infiltration in their BM (11%, 31%, 31%, and 41%) and spleen (24%, 42%, 66%, and 79%), respectively. (C) Spleen weights were determined for mice euthanized after relapse in the trial shown in panel B. Despite the presence of normal spleen weights in mice that received the 3-drug combination, animals nonetheless died of leukemia after the limited 13-day drug exposure. (D) Mice were treated continuously twice daily with dasatinib alone (10 mg/kg) or together with ruxolitinib (60 mg/kg) with or without dexamethasone as indicated. Therapy in the group receiving the 3-drug combination was terminated after 6 months; surviving animals were observed for signs of disease and subjected to intermittent bioluminescence imaging for an additional 200 days. Significance for pairwise comparisons (not including 2 surviving mice) is dasatinib alone vs dasatinib plus ruxolitinib (P = .029); dasatinib plus ruxolitinib vs dasatinib plus ruxolitinib plus dexamethasone (P < .0002); dasatinib alone vs dasatinib plus ruxolitinib plus dexamethasone (P < .0001).
Ruxolitinib and dexamethasone each prolong remission in dasatinib-treated mice. All mice were infused intravenously with 2 × 105 LICs. Mice that were left untreated or received drugs 10 days later were followed until they became moribund. (A) Mice treated for 5 days per week once per day by oral gavage with ruxolitinib alone (60 mg/kg body weight) exhibited no survival advantage compared with untreated or vehicle-treated mice, whereas mice receiving ruxolitinib plus dasatinib (10 mg/kg body weight) on the same dosing schedule showed a significant survival advantage over animals receiving dasatinib monotherapy (P < .001). (B) Mice were treated continuously for 13 days with the indicated drug combinations, after which therapy was discontinued. Dasatinib (10 mg/kg) was administered twice per day (unlike the once per day regimen in panel A) and ruxolitinib (60 mg/kg) administered once per day, each by oral gavage. Dexamethasone was added, as indicated, to the drinking water. The time to treatment failure (abscissa) reflects the level of MRD at the end of day 22. TaqMan PCR performed at necropsy on 4 animals that relapsed after combined treatment with dasatinib and ruxolitinib showed significant leukemic infiltration in their BM (11%, 31%, 31%, and 41%) and spleen (24%, 42%, 66%, and 79%), respectively. (C) Spleen weights were determined for mice euthanized after relapse in the trial shown in panel B. Despite the presence of normal spleen weights in mice that received the 3-drug combination, animals nonetheless died of leukemia after the limited 13-day drug exposure. (D) Mice were treated continuously twice daily with dasatinib alone (10 mg/kg) or together with ruxolitinib (60 mg/kg) with or without dexamethasone as indicated. Therapy in the group receiving the 3-drug combination was terminated after 6 months; surviving animals were observed for signs of disease and subjected to intermittent bioluminescence imaging for an additional 200 days. Significance for pairwise comparisons (not including 2 surviving mice) is dasatinib alone vs dasatinib plus ruxolitinib (P = .029); dasatinib plus ruxolitinib vs dasatinib plus ruxolitinib plus dexamethasone (P < .0002); dasatinib alone vs dasatinib plus ruxolitinib plus dexamethasone (P < .0001).
All mice treated as in Figure 4A succumbed to leukemia, in many cases accompanied by hind limb paralysis, doming of the head, and intermittent generalized seizures, implicating meningeal or CNS involvement.23,29 Although dasatinib penetrates the blood-brain barrier, an active efflux of the drug from brain capillary endothelial cells into the blood via 2 different exporters limits drug delivery to the CNS and impedes systemic therapy.30,31 Histopathologic examination of tissues from moribund mice confirmed significant leukemic infiltration of the meninges in all animals, whether they failed due to CNS-centered or more disseminated disease (supplemental Figure 2). Therefore, the CNS is a “safe haven” for Ph+ ALL cells that efficiently escape from combined treatment with dasatinib and ruxolitinib.
Given the efficacy of dexamethasone during induction therapy (Figure 3) and its ability to cross the blood-brain barrier and reduce the risk of CNS relapse,23 it was incorporated into the treatment regimen. Initially, mice infused with LICs were again randomized to different treatment groups at day 10 and treated with various drug combinations for only 13 days. Bioluminescence imaging was used to verify disease remission after therapy, and then “time to treatment failure” (ie, time to terminal leukemia relapse after drug withdrawal) was evaluated (Figure 4B). We reasoned that differences in survival after treatment termination should serve as a surrogate parameter for the level of residual disease achieved by different treatment schemes. Although dexamethasone alone or with ruxolitinib provided no significant survival advantage, both agents improved the duration of remission in response to dasatinib (Figure 4B). However, disseminated leukemia documented by increasing bioluminescence, clinical criteria, and qPCR (Figure 4B, legend) invariably ensued. Ruxolitinib and dexamethasone each offered additive benefits, indicating that the 3 drugs act through different mechanisms in extending survival. Despite additional immunosuppressive effects of dexamethasone, we did not observe infectious complications. The combination of dasatinib and ruxolitinib led to a significant reduction of spleen weights in moribund mice, an effect enhanced by dexamethasone (Figure 4C). Continuous treatment with the 3-drug regimen for up to 6 months led to a further beneficial effect in leukemic mice, significantly improving their survival during and after therapy (Figure 4D; see figure legend for P values). Indeed, 2 of 12 mice that received continuous therapy for 6 months were alive and healthy 6 months after discontinuation of treatment.
The combination of 3 targeted agents selects for fewer BCR-ABL mutations at relapse
For the long-term treatment study shown in Figure 4D, we used PCR amplification and nucleotide sequencing to evaluate genomic DNA from treated and untreated mice for the presence of BCR-ABL KD mutations. From each treatment cohort, 5 to 7 moribund mice that underwent relapse, as well as 5 untreated leukemic animals (from Figure 4B) were euthanized. The genomic DNA obtained from BM of individual animals was analyzed. No BCR-ABL KD mutations were detected in samples obtained from untreated leukemic mice (Table 1). In contrast, we observed a strong selective effect of dasatinib alone, or dasatinib plus ruxolitinib, for the occurrence of KD mutations. Three mice developed leukemic clones harboring the T315I or the F317L mutations, both of which inhibit dasatinib binding; others developed P-loop mutations that affect kinase activity and transformation potency without directly interfering with TKI binding.32-34 Remarkably, the small cohort of 5 leukemic mice that received dasatinib, ruxolitinib, and dexamethasone displayed no BCR-ABL KD mutations at relapse (P < .015), implying that therapeutic failure need not depend on this resistance mechanism.
Identity and frequency of BCR-ABL kinase mutations in relapsed leukemia
| Relapse BM sample . | Treatment group (% mutant clones) . | |||
|---|---|---|---|---|
| None . | Dasa . | Dasa + Rux* . | Dasa + Rux + Dex* . | |
| 1 | 0 | Y253F (36)† | T315I (100) | 0 |
| 2 | 0 | T315I (100) | E255K (100) | 0 |
| 3 | 0 | F317L (92) | E255K (100) | 0 |
| 4 | 0 | 0 | E255K (43) | 0 |
| 5 | 0 | 0 | Q252H (23) | 0 |
| 6 | ND | E255K (36) | F401L (25) | ND |
| 7 | ND | ND | 0 | ND |
| Relapse BM sample . | Treatment group (% mutant clones) . | |||
|---|---|---|---|---|
| None . | Dasa . | Dasa + Rux* . | Dasa + Rux + Dex* . | |
| 1 | 0 | Y253F (36)† | T315I (100) | 0 |
| 2 | 0 | T315I (100) | E255K (100) | 0 |
| 3 | 0 | F317L (92) | E255K (100) | 0 |
| 4 | 0 | 0 | E255K (43) | 0 |
| 5 | 0 | 0 | Q252H (23) | 0 |
| 6 | ND | E255K (36) | F401L (25) | ND |
| 7 | ND | ND | 0 | ND |
Cohorts of untreated leukemic mice (Figure 4B) or from mice undergoing relapse during continuous treatment with dasatinib (Dasa), ruxolitinib (Rux), and dexamethasone (Dex) (Figure 4D) were euthanized when moribund. BM cells from 5 to 7 individual leukemic mice from each group were harvested from their long bones, genomic DNA was extracted, and BCR-ABL KD sequences were amplified from 200 ng of DNA using nested PCR. Individual PCR products were subcloned into plasmids transfected into Escherichia coli, and bacteria were streaked onto plates containing isopropyl β-D-1-thiogalactopyranoside to form individual recombinant colonies, 10 to 20 of which were picked at random from each such sample. Recloned BCR-ABL KD DNAs were sequenced in both directions to avoid sequencing errors.
ND = not done.
Differences between the Dasa + Rux and Dasa + Rux + Dex cohorts are significant (P = 0.015, Fisher’s 2-tailed exact test).
Mutations identified in 20% or more of the sequenced clones are designated in single-letter amino acid code (with mutational frequencies in parenthesis). For example, “Y253F (36)” means that a mutation encoding a phenylalanine for tyrosine substitution at codon 253 was identified in 5 of 14 clones (36%) from a single leukemic BM sample. In no case was more than a single KD mutation detected in a relapse sample. T315I and F317L are gatekeeper mutations that prevent dasatinib binding; Y253F, E255K, and Q252H are P-loop mutations. F401L has not been characterized.
Discussion
BCR-ABL expression and disruption of the CDKN2A/B and IKZF1 (IKAROS) loci are the key genetic alterations in the pathogenesis of human Ph+ ALL.1,6,7 The short-term culture conditions used in our experiments arrest B-cell development at the pro-/pre-B-cell stage,25 mimicking effects of IKZF1 mutations8,9 and making BCR-ABL expression and Arf inactivation sufficient to guarantee leukemogenesis.11 Notably, BCR-ABL-positive, Arf−/− LICs generate ALL in healthy nonirradiated, syngeneic mice, modeling human ALL that arises in immunocompetent hosts. Coexpression of the Luc2 gene with BCR-ABL in donor LICs enables the visualization and quantification of leukemia burden over at least a 3-log range, allowing sequential monitoring of disease progression by noninvasive means. Bioluminescent imaging permits accurate synchronization of disease stage in leukemic mice prior to treatment, controlling for genetic differences in donor cells (eg, γC positive or negative) and experimental variations in the injection technique, and allowing valid randomization of recipient mice with very similar disease burdens to different treatment cohorts. Moreover, inclusion of the Luc2 gene allows independent and highly sensitive qPCR-based estimates of leukemia burden and of differential therapeutic responses during induction therapy, a time when the intensity of bioluminescence images rapidly becomes negligible.
The improved response to TKIs in mice inoculated with LICs that lacked the common γC points directly to salutary effects of cytokines in supporting the viability of LICs in the face of dasatinib treatment. Ruxolitinib mimicked effects of abrogated cytokine signaling caused by the γC knockout but had no antileukemic effects when administered alone and was unable to augment the immediate effects of dasatinib in rapidly decreasing the leukemia burden during the initial phase of induction therapy. However, when combined with more prolonged dasatinib treatment, which was predicted to reinstate cytokine dependence, ruxolitinib proved effective. In short, effective dasatinib therapy sets a precondition in which ruxolitinib acquires therapeutic efficacy in maintaining disease remission. Recent work of others has suggested that addition of ruxolitinib to TKI therapy might also improve therapeutic efficacy in CML.35 Combining these 2 agents facilitated the apoptotic elimination of cultured human CML CD34+ cells and prevented their engraftment into immunodeficient mice. Unfortunately, untreated transplanted CML cells were not leukemogenic, limiting any possibility of in vivo treatment trials.35
The time-to-treatment-failure approach, following a relatively brief 13-day drug exposure, provides a cost-efficient means to evaluate therapeutic options in our model and can be used as a surrogate parameter for MRD status due to the highly predictable population doubling time of untreated leukemic cells in vivo (∼20 hours11 ). Strikingly, 13-day monotherapy of leukemic mice with twice-daily dasatinib begun 10 days after LIC infusion produced at least a 4-log reduction in leukemic burden in BM and spleen, and yet all continuously treated animals underwent relapse. Even though dasatinib penetrates the blood-brain barrier, its efficacy is limited by active efflux from brain capillary endothelia into the blood.30,31 Remissions achieved with the combination of dasatinib and ruxolitinib were more durable, but mice still relapsed, and many exhibited clinical symptoms and signs of CNS involvement. Dexamethasone, a cornerstone of ALL therapy that improves control of leukemia in the CNS,23 further extended progression-free survival. In a cohort that received 6-month treatment with this 3-drug combination, a small subset of leukemic mice lived for an additional 6 months after discontinuation of therapy. The efficacy of this treatment scheme was not diminished by toxicity or more frequent infectious complications, reflecting the benefit of targeted therapy in combination with steroids compared to “conventional” high-dose chemotherapy.
By maintaining a reservoir of LICs that can undergo additional genetic alterations in the face of continued drug treatment, MRD can allow the emergence of clones with BCR-ABL KD mutations that confer resistance to TKI therapy and lead to leukemic relapse. BCR-ABL mutations emerging during therapy depend not only on the duration and intensity of therapy as shown here and in previous studies with this model12 but also on the therapeutic agents used. Although combined treatment with dasatinib and ruxolitinib yielded many mutant clones at relapse, addition of dexamethasone significantly reduced their overall frequency. Therefore, the emergence of BCR-ABL mutations is not requisite for relapse, and treatment with broadly active third-generation TKIs that inhibit even gatekeeper mutations may not be sufficient to cure Ph+ ALL.36 The mechanisms that confer drug resistance to combinatorial therapy with dasatinib, ruxolitinib, and dexamethasone remain unclear. However, more than 15 different kinase-activating mutations, all of which invoke a BCR-ABL-like program, were identified in 91% of patients with Ph-like ALL,37 implying that a wide variety of resistance mechanisms might restore leukemogenesis in response to targeted therapy. Clearly, the best way to treat Ph+ ALL is to minimize or eliminate MRD.4
In summary, our data underscore the relevance of this preclinical model and further elucidate the key role of the hematopoietic microenvironment in maintaining MRD. Taken together, our results point to a new therapeutic strategy for the treatment of Ph+ ALL and provide a rationale for a phase 1/2 clinical trial in which patients with Ph+ ALL age ≥40 years will receive dasatinib, ruxolitinib, dexamethasone, and intrathecal methotrexate as a first-line remission induction therapy for 12 weeks, followed by continuation of therapy or other treatment options, including conventional chemotherapy or allogeneic stem cell transplant for eligible patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Dr Mildred Scheel postdoctoral fellowship of the German Cancer Aid (I.A.), the Leukemia & Lymphoma Society Fellow Award 5428-15 (C.D.R.), a Career Development Program Award 5099-12 (C.C.), and a Specialized Center of Research grant 7015-09 (S.W.L.). This work was also supported by a National Institutes of Health, National Cancer Institute grant P30 CA 008748 (E.d.S.), a US Public Health Service Cancer Center (CORE) grant CA-21765 (C.J.S.), and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital (C.J.S.). S.W.L and C.J.S. are Howard Hughes Medical Institute investigators.
Authorship
Contribution: I.A., C.D.R., and C.J.S. designed the experiments; I.A., C.D.R., E.d.S., C.C., and G.C. performed the work; S.W.L. provided suggestions on the experimental protocols and helped support the work; and I.A., C.D.R., and C.J.S. formulated the data for publication and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for I.A. is Clinic for Oncology, Hematology, and Stem Cell Transplantation, University Hospital Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen, Aachen, Germany.
Correspondence: Charles J. Sherr, Department of Tumor Cell Biology, St Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105; e-mail: Charles.Sherr@stjude.org.
References
Author notes
I.A. and C.D.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal