Key Points
TOX is aberrantly expressed in primary Sézary cells and its levels correlate with increased risk of disease-specific mortality.
TOX knockdown promotes apoptosis and reduces cell proliferation in CTCL cells, partially through inducing p27 and p57.
Abstract
TOX is a nuclear factor essential for the development of CD4+ T cells in the thymus. It is normally expressed in low amounts in mature CD4+ T cells of the skin and the peripheral blood. We have recently discovered that the transcript levels of TOX were significantly increased in mycosis fungoides, the most common type of cutaneous T-cell lymphoma (CTCL), as compared to normal skin or benign inflammatory dermatoses. However, its involvement in advanced CTCL and its biological effects on CTCL pathogenesis have not been explored. In this study, we demonstrate that TOX expression is also enhanced significantly in primary CD4+CD7− cells from patients with Sézary syndrome, a leukemic variant of CTCL, and that high TOX transcript levels correlate with increased disease-specific mortality. Stable knockdown of TOX in CTCL cells promoted apoptosis and reduced cell cycle progression, leading to less cell viability and colony-forming ability in vitro and to reduced tumor growth in vivo. Furthermore, TOX knockdown significantly increased 2 cyclin-dependent kinase (CDK) inhibitors, CDKN1B and CDKN1C. Lastly, blocking CDKN1B and CDKN1C reversed growth inhibition of TOX knockdown. Collectively, these findings provide strong evidence that aberrant TOX activation is a critical oncogenic event for CTCL.
Introduction
Cutaneous T-cell lymphoma (CTCL) consists of 2 main subtypes: mycosis fungoides (MF), which primarily affects the skin; and Sézary syndrome (SS), which is characterized by the presence of circulating malignant Sézary cells. Together, MF and SS account for 65% to 80% of CTCL cases.2-4 In both conditions, there is accumulation of malignant mature CD4+CD45RO+ T cells. Although the etiology of CTCL is not yet clear, accumulating evidence indicates that defects in apoptosis and cell cycle control are critical in disease pathogenesis.5-8 However, the molecular events leading to these abnormalities have not been well understood.
By transcriptome analysis, we have recently demonstrated that the transcript levels of thymocyte selection-associated high-mobility group (HMG) box gene (TOX) are abnormally increased in early-stage MF skin biopsy specimens but not in the biopsy specimens of benign inflammatory dermatoses (BID) or normal skin. Specific TOX antibodies identified the malignant CD4+ T cells in the dermis and epidermis of MF, including the Pautrier microabscesses, the pathologic hallmark of MF.9 This finding was recently confirmed independently by 2 research groups.10,11 Emerging evidence has pointed to SS and MF having different cellular origin and expression profiling.12 It is unknown whether SS also contains ectopic TOX expression.
TOX is a nuclear protein essential for the proper development of thymocytes. Mice deficient in TOX failed to develop CD4+ T-cell lineage; however, on the newly formed CD4+ T cells exiting the thymus, TOX is tightly downregulated and remains suppressed in the mature CD4+ T cells in the skin and blood.13-15 Therefore, the observation of TOX expression in MF skin biopsy specimens is highly unusual. Whether TOX contributes to CTCL pathogenesis has not been investigated.
In this study, we systematically examined whether TOX aberrant expression was also present in SS and cultured CTCL cell lines. Further, we performed in vitro and in vivo experiments to define the pathogenic significance of TOX in CTCL oncogenesis. We also explored the possible downstream mediators of TOX in CTCL. We demonstrate that TOX aberrant expression is a feature shared by both SS and MF; that TOX overexpression plays an oncogenic role in vitro and in vivo; and that TOX does so partially through disrupting the transcription of 2 cyclin-dependent kinase (CDK) inhibitors, CDKN1B and CDKN1C. These findings argue that TOX is a novel oncogene for CTCL.
Materials and methods
Human primary cells and established T-cell lymphoma cell lines
The Sézary cells (CD4+CD7− T cells) from SS patients (see supplemental Table 1, available on the Blood Web site), control benign cells, normal CD4+ T cells, and their preparation details have been described previously.16,17 Every participant gave informed consent. The procedures used were in accordance with the Declaration of Helsinki principles and approved by the Research Ethics Board of the University of British Columbia and the Mayo Clinic College of Medicine.
Human CTCL cell lines Hut78, HH (ATCC no. TIB-161, CRL-2105), and SZ4 (a generous gift from Dr James Herman at Johns Hopkins University) were cultured in full RPMI 1640 containing 10% fetal bovine serum, 100 U/mL of penicillin, 0.1 mg/mL of streptomycin, and 10−4 M β-mercaptoethanol (STEMCELL Technologies, Vancouver, BC, Canada). Transduced-CTCL cells were cultured in the above medium plus puromycin or hygromycin or a combination of both (Invitrogen, Burlington, ON, Canada).
RNA extraction and qRT-PCR
Total RNA was extracted and reverse transcribed; quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed and analyzed as described previously.9,18-20 Gene expression levels were expressed as mRNA copies per 1000 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) copies. qRT-PCR primers are listed in supplemental Table 2.
Activation of CD4+ T cells
Peripheral blood CD4+ T cells (STEMCELL Technologies) were activated by phorbol 12-myristate 13-acetate and ionomycin (Sigma-Aldrich, St Louis, MO) as described previously17,21,22 or by mouse anti-human CD3 antibody with or without mouse anti-human CD28 antibody (BD Biosciences, Mississauga, ON, Canada) as described previously.17,23 An isotope control antibody (BD Biosciences) was also used to estimate nonspecific binding.
Western blot analysis and antibodies
Protein was extracted, quantified, and assessed by western blot analysis as described previously.17,18 Protein lysates were probed with rabbit anti-TOX antibody (Sigma-Aldrich), rabbit anti-p27 (C-19; Santa Cruz Biotechnology, Paso Robles, CA), rabbit anti-p57 (C-20; Santa Cruz Biotechnology), rabbit anti-caspase 3 (Cell Signaling Technology), and rabbit anti-caspase 9 (Cell Signaling Technology). Mouse antibody against human actin (Sigma-Aldrich) was included as a loading control.
FACS analysis
Fluorescence-activated cell sorter (FACS) data were collected by a FACSCanto II or a Fortessa cell analyzer (BD Biosciences), and analyzed with FloJo 7.6 software (Tree Star, Ashland, OR). For human peripheral blood, cells in the lymphocyte gate were used for analysis of CD4 and TOX status using rabbit anti-human TOX (phycoerythrin-conjugated, eBioscience, San Diego, CA) and mouse anti-human CD4 (APC-eFluor 780–conjugated, eBioscience).
Immunofluorescence staining
Immunofluorescence staining was performed in cell pellets as previously described.9 Images were collected under a Zeiss AxioVert 200M inverted fluorescence microscope (Carl Zeiss AG, Jena, Germany), and processed with the Zeiss AxioVision 4.8 image acquisition and processing software (Carl Zeiss AG). Brightness and contrast were adjusted consistently across all images.
Lentivirus generation and gene knockdown
pLKO.1 puro vector containing short hairpin (sh)RNA inserts that specifically target human TOX (SHCLNG-XM_376776; Sigma-Aldrich) and a nontargeting shRNA control (SHC002; Sigma-Aldrich) were purchased, whereas shRNA inserts that specifically target human CDKN1B and CDKN1C and a nontargeting shRNA control were cloned into pLKO.1 hygro backbone, a kind gift from Bob Weinberg (Addgene plasmid #24150). The oligonucleotides encoding the shRNAs are included in supplemental Table 2. The shRNA inserts were verified by Sanger sequencing. Lentiviral particles were generated by transfecting HEK 293T packaging cells using the polyethylenimine method as described previoiusly.24 After 48 and 72 hours, the virus-containing medium (35 mL per construct) was filtered and concentrated in 200 μL of RPMI 1640 medium. CTCL cells (2 × 105 cells in 1 mL of media) were incubated with 5 μL of concentrated lentiviral particles for 24 hours. After another 24 hours, puromycin or hygromycin was added to select transduced populations. TOX, p27, and p57 protein levels were evaluated by western blot analysis 5 to 7 days after puromycin or hygromycin selection. Bulk populations transduced either by control (CTR) or shRNA viruses were used in this study.
Viability assay
For single TOX knockdown experiments, transduced CTCL cells were cultured in 12-well plates (2.5 × 105 cells per well) containing 2 mL of full RPMI 1640 medium with puromycin (1 μg/mL). For co-knockdown of TOX and CDKN1B or CDKN1C experiments, transduced CTCL cells were cultured in 12-well plates (1.5 × 105 cells per well) containing 2 mL of full RPMI 1640 medium with puromycin (1 μg/mL) and hygromycin (750 μg/mL for Hut78; 200 μg/mL for HH). Cells were incubated at 37°C for 24, 48, 72, and 96 hours. At each time point, 10 μL of cell mixture from each well was used to determine viable cell numbers by the trypan blue exclusion method. Each population was plated in duplicate or triplicate wells, and at least 3 biological replicates were performed for each cell line.
CFC assay
Colony-forming cell (CFC) assays were performed in fetal calf serum–containing methylcellulose cultures (H4230; STEMCELL Technologies) in the presence of 1 μg/mL of puromycin (Invitrogen). Colony counts were performed using standard scoring criteria after 12 to 14 days of incubation.16,18 Colony size was determined by cell number per colony (big, >500 cells; medium, 50-500 cells; small, 20-50 cells).
Mouse xenograft models of CTCL
Mice were bred and maintained at the British Columbia Cancer Research Center Animal Facility. All experimental protocols were approved by the University of British Columbia Animal Care Committee. NOD/scid interleukin-2 receptor γ-chain–deficient (NSG) mice at 8 weeks of age were injected subcutaneously on both flanks with 1 × 106 transduced Hut78 or HH cells (CTR, n = 6; TOXsh-1, n = 6; TOXsh-2, n = 6). Mice were monitored for local tumor formation 3 times a week, and tumor sizes were measured with a glide caliper. Tumor volume was calculated using the formula (length [mm] × width [mm]2) ÷ 2.25
H&E staining and immunohistochemistry staining
Mouse tissues were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). For immunohistochemistry staining, mouse tissues were probed with rabbit monoclonal anti-human CD3 antibody (SP7; Spring Bioscience, Pleasanton, CA) and UltraMap anti-rabbit horseradish peroxidase–conjugated secondary antibody (Ventana Medical Systems, Tucson, AZ), following procedures described previously.26
Apoptosis assay
Apoptosis assays were performed using an apoptosis detection kit (BD Biosciences) as previously described.27 Total apoptotic cell numbers were calculated as total annexin V–positive cells.
BrdU incorporation assay
5-Bromo-2′-deoxyuridine (BrdU) incorporation assays were performed using an FITC BrdU Flow Kit (BD Biosciences) following the manufacturer’s instructions. Transduced Hut78 cells were pulsed with 10 μM BrdU for 45 minutes, whereas transduced HH and SZ4 cells were pulsed for 2 hours.
Statistical analysis
GraphPad Prism 5.00 (San Diego, CA) and X-tile (New Haven, CT) software programs were used for statistical analyses. Continuous variables were compared by 2-tailed Student t tests. The receiver operating characteristic method was used to determine whether TOX mRNA levels could differentiate SS from BID and healthy participants (HPs). Disease-specific mortality was assessed using the Kaplan-Meier curve and log-rank test. We determined the optimal cutpoint (lowest P value) of TOX mRNA expression levels as 129.9 using the X-tile software.28 In our analysis, TOX high and TOX low groups were defined by the expression levels higher and lower than 129.9, respectively. P values <.05 were considered statistically significant.
Results
TOX is ectopically upregulated in primary CD4+CD7− cells from SS patients
To determine transcript levels of TOX in SS, we compared expression of TOX in CD4+CD7− T cells purified from the peripheral blood of 12 SS patients to that in benign CD4+ T cells from 18 BID patients (psoriasis, n = 8; rosacea, n = 5; vitiligo, n = 5) or 9 HPs. Because BID and HP cells expressed similar transcript levels of TOX (33.66 ± 3.17 vs 44.75 ± 7.64, respectively; P = .21), they were combined as the non-SS controls. Compared to non-SS, SS CD4+CD7− T cells expressed significantly higher TOX mRNA (fivefold; P = .012; Figure 1A). Among the 12 SS samples, SS with T-cell receptor (TCR) clonality (n = 7) possessed more TOX mRNA (489.3 ± 138.7), compared to SS without TCR clonality (n = 5; 172.5 ± 51.87; P = .070; Figure 1B).
TOX mRNA levels are increased in peripheral blood CD4+ T cells from SS patients and correlate with disease-specific mortality. (A) TOX mRNA levels in peripheral blood CD4+ T cells from SS patients (n = 12), HPs (n = 9), and BID patients (psoriasis [PSO], n = 8; rosacea [ROS], n = 5; vitiligo [VT], n = 5). In each sample, RNA was extracted as described in the “Methods and materials” section. qRT-PCR was performed using primers specific for TOX and GAPDH mRNA. The level of TOX mRNA was normalized to that of GAPDH so that the levels shown represent copies of TOX mRNA per 1000 copies of GAPDH mRNA. (B) TOX mRNA levels in CD4+ T cells from SS patients with clonality (n = 7) vs without clonality (n = 5). (C) Receiver operating characteristic curve of TOX mRNA levels in distinguishing SS from non-SS. (D) High TOX mRNA levels in peripheral CD4+ T cells correlated with a higher disease-specific mortality. TOX high and TOX low groups were defined by the expression levels higher and lower than 129.9, respectively. AUC, area under the curve.
TOX mRNA levels are increased in peripheral blood CD4+ T cells from SS patients and correlate with disease-specific mortality. (A) TOX mRNA levels in peripheral blood CD4+ T cells from SS patients (n = 12), HPs (n = 9), and BID patients (psoriasis [PSO], n = 8; rosacea [ROS], n = 5; vitiligo [VT], n = 5). In each sample, RNA was extracted as described in the “Methods and materials” section. qRT-PCR was performed using primers specific for TOX and GAPDH mRNA. The level of TOX mRNA was normalized to that of GAPDH so that the levels shown represent copies of TOX mRNA per 1000 copies of GAPDH mRNA. (B) TOX mRNA levels in CD4+ T cells from SS patients with clonality (n = 7) vs without clonality (n = 5). (C) Receiver operating characteristic curve of TOX mRNA levels in distinguishing SS from non-SS. (D) High TOX mRNA levels in peripheral CD4+ T cells correlated with a higher disease-specific mortality. TOX high and TOX low groups were defined by the expression levels higher and lower than 129.9, respectively. AUC, area under the curve.
Enhanced transcript levels of TOX correlate with increased risk of disease-specific mortality in SS
We next examined whether TOX levels could differentiate SS from non-SS using the receiver operating characteristic curve, and whether TOX levels correlated with patient survival using a Kaplan-Meier plot and log-rank test. Increased TOX mRNA levels had an area-under-the-curve value of 0.824 (P = .001), indicating that TOX could be useful in improving the diagnosis of SS (Figure 1C). Furthermore, a strong correlation was found between higher TOX mRNA levels and increased SS-related death (P = .039; log-rank test hazard ratio, 5.68; Figure 1D). SS patients with higher TOX mRNA levels (n = 6; >129.9) had a much shorter median survival time (27 months) than that of SS with TOX mRNA levels lower than 129.9 (n = 6; 120 months).
T-cell activation suppresses TOX expression in normal mature CD4+ T cells but not in CTCL cells
TOX is highly induced by pre-TCR activation in thymocyte precursors.13 In addition, lymphocyte activation is involved in the pathogenesis of CTCL.29 We therefore asked whether the observed TOX increase was due to continuous cellular activation in normal CD4+ T cells. T-cell activation by 25 ng/mL of phorbol 12-myristate 13-acetate and 50 ng/mL of ionomycin, or by anti-human CD3 antibody (± anti-human CD28 antibody) decreased TOX levels by twofold to 13-fold (P < .05; supplemental Figure 1A) in normal CD4+ T cells. However, this effect was impaired in Hut78 cells and absent in HH and SZ4 cells (supplemental Figure 1B).
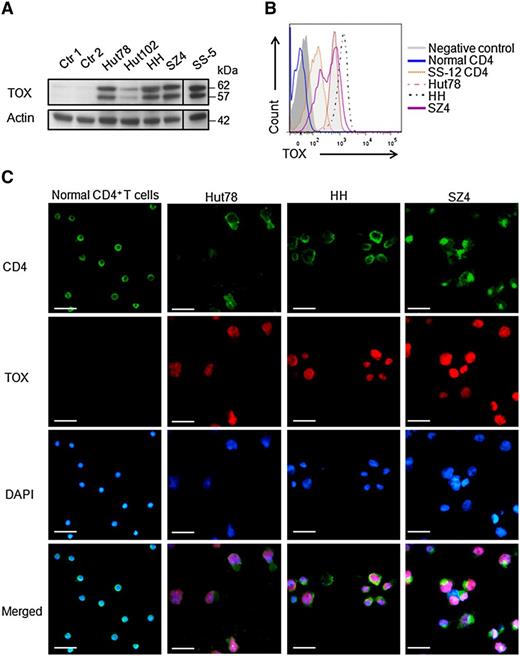
TOX protein is overexpressed in primary Sézary cells and in multiple CTCL cell lines
In addition to transcript level, TOX protein levels were also highly increased in primary Sézary cells from an SS patient (SS-5) and in 4 CTCL cell lines (Hut78, HH, Hut102, and SZ4), as compared to CD4+ cells from BID patients or HPs by western blot analysis (Figure 2A). This finding demonstrated that highly deregulated expression of TOX was found not only in MF skin biopsy specimens as we previously reported9 but also in Sézary cells. Of note, Hut102, derived from MF (early CTCL), expressed much lower TOX protein compared to the more advanced CTCL cell lines (Figure 2A), derived from either SS (Hut78, SZ4) or non-MF/SS aggressive CTCL (HH), suggesting that overexpressed TOX may contribute to the disease progression. We further confirmed the TOX protein overexpression by FACS analysis. Compared to normal CD4+ lymphocytes (TOX level similar to negative control), CD4+ lymphocytes from an SS patient (SS-12) and Hut78, HH, and SZ4 cells expressed much higher levels of TOX protein (Figure 2B). Similarly, immunofluorescence staining in 3 CTCL cell lines showed strong nuclear staining of TOX, whereas none was detected in normal CD4+ T cells (Figure 2C).
TOX protein levels are increased in SS CD4+ T cells and in CTCL cell lines. (A) Western blot analysis showed TOX overexpression in 4 CTCL cell lines (Hut78, Hut102, HH, and SZ4) and in CD4+ T cells from the peripheral blood of a patient with SS (SS-5), compared to peripheral CD4+ T cells from an HP (Ctr 1) or a patient with BID (Ctr 2). For each sample, cell lysate was extracted as described in the “Methods and materials” section and probed with antibodies specific for TOX and actin proteins. (B) FACS analysis showed TOX elevation in the peripheral CD4+ T cells from a patient with SS (SS-12) and in the CTCL cell lines Hut78, HH, and SZ4, whereas normal CD4+ T cells were negative for TOX staining. A fluorescence-minus-one control was included as a negative control. (C) Immunofluorescence staining showed strong nuclear TOX staining in the CTCL cells lines Hut78, HH, and SZ4 but not in normal CD4+ T cells. For each cell type, 1 million cells were mounted onto the slide and subjected to staining as described in the “Methods and materials” section. Bars represent 25 μm. DAPI, 4′,6 diamidino-2-phenylindole.
TOX protein levels are increased in SS CD4+ T cells and in CTCL cell lines. (A) Western blot analysis showed TOX overexpression in 4 CTCL cell lines (Hut78, Hut102, HH, and SZ4) and in CD4+ T cells from the peripheral blood of a patient with SS (SS-5), compared to peripheral CD4+ T cells from an HP (Ctr 1) or a patient with BID (Ctr 2). For each sample, cell lysate was extracted as described in the “Methods and materials” section and probed with antibodies specific for TOX and actin proteins. (B) FACS analysis showed TOX elevation in the peripheral CD4+ T cells from a patient with SS (SS-12) and in the CTCL cell lines Hut78, HH, and SZ4, whereas normal CD4+ T cells were negative for TOX staining. A fluorescence-minus-one control was included as a negative control. (C) Immunofluorescence staining showed strong nuclear TOX staining in the CTCL cells lines Hut78, HH, and SZ4 but not in normal CD4+ T cells. For each cell type, 1 million cells were mounted onto the slide and subjected to staining as described in the “Methods and materials” section. Bars represent 25 μm. DAPI, 4′,6 diamidino-2-phenylindole.
Stable knockdown of TOX inhibits growth of CTCL cells in vitro
To investigate whether knockdown of TOX expression would affect cell growth of CTCL cells, 3 CTCL cells lines (Hut78, HH, and SZ4) were transduced with lentiviruses containing either a nontargeting control sequence (CTR) or each of 2 shRNA sequences against human TOX. Knockdown of TOX (>90%) by 2 shRNA constructs (sh-1 and sh-2) was confirmed in all 3 CTCL cell lines by western blot analysis (Figure 3A). Stable suppression of TOX resulted in significant reduced cell growth in all 3 cell lines compared to control cells (approximately twofold to fourfold; P < .001; Figure 3B). Similarly, TOX suppression resulted in marked reduction in CFC output in all 3 cell lines. In addition to reduction in colony numbers, the colonies generated by TOX-sh cells were much smaller and more dispersed than those generated by the control cells (Figure 3C).
TOX suppression confers growth disadvantage to CTCL cells. (A) TOX knockdown by 2 shRNA (sh-1 and sh-2, both specific for TOX mRNA) compared to control (CTR) CTCL cells transduced by a nontargeting shRNA. Transduced cells were selected by puromycin (1 μg/mL) for 5 days before protein lysates were probed with antibodies against TOX and actin proteins. (B) TOX-sh cells had a much reduced viable cell number over a period of 4 days compared to CTR cells. A total of 2.5 × 105 cells were cultured in 2 mL of full RPMI media for 4 days, and viable cells were determined each day by the trypan blue exclusion method. (C) TOX-sh cells generated fewer and smaller colonies in 3-dimensional culture compared to CTR cells. Three hundred cells were plated in 1.5 mL of methycellulose, and colony numbers and morphology were recorded on day 12. Numbers of colonies of different sizes (big, medium, and small) are depicted. Photographs of representative colonies are also shown. Original magnification ×10. **P < .01 and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data shown here are representative of at least 3 independent experiments.
TOX suppression confers growth disadvantage to CTCL cells. (A) TOX knockdown by 2 shRNA (sh-1 and sh-2, both specific for TOX mRNA) compared to control (CTR) CTCL cells transduced by a nontargeting shRNA. Transduced cells were selected by puromycin (1 μg/mL) for 5 days before protein lysates were probed with antibodies against TOX and actin proteins. (B) TOX-sh cells had a much reduced viable cell number over a period of 4 days compared to CTR cells. A total of 2.5 × 105 cells were cultured in 2 mL of full RPMI media for 4 days, and viable cells were determined each day by the trypan blue exclusion method. (C) TOX-sh cells generated fewer and smaller colonies in 3-dimensional culture compared to CTR cells. Three hundred cells were plated in 1.5 mL of methycellulose, and colony numbers and morphology were recorded on day 12. Numbers of colonies of different sizes (big, medium, and small) are depicted. Photographs of representative colonies are also shown. Original magnification ×10. **P < .01 and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data shown here are representative of at least 3 independent experiments.
TOX suppression impairs tumor-forming ability of CTCL cells in vivo
To investigate whether deregulation of TOX in CTCL cells contributes to their ability to induce tumor in vivo, we injected transduced CTCL cells (Hut78 and HH) into NSG mice and monitored local tumor formation. (TOX knockdown resulted in rapid death of SZ4 cells, which could not be assayed in the same fashion). Mice injected with Hut78 CTR or HH CTR (n = 6) cells formed local tumors within 13 days; the tumors grew progressively and reached the maximum allowed tumor volume of 1700 mm3 (by 23 days or 30 days, respectively). In contrast, only 1 of 6 mice injected with Hut78 TOX-sh cells and 5 of 6 mice injected with HH TOX-sh cells formed tumors, which were significantly smaller than the control tumors (Figure 4A,Bi-ii). Local tumors formed by CTR cells showed abnormal mitosis and more extensive destruction of subcutaneous tissues compared to TOX-sh cells (H&E staining, Figure 4Biii). The infiltrating tumor cells were positive for human CD3, confirming their origin in human helper T cells (Figure 4Biv). These findings indicate that stable suppression of TOX expression profoundly impairs the transforming ability of CTCL cells in vivo.
TOX inhibition impairs the tumor-forming ability of CTCL cells in vivo. (A) Tumor volume comparison between Hut78 and HH mice injected with 1 million TOX-sh cells (n = 12) or control (CTR) cells (n = 6). Mice were monitored for local tumor formation 3 times a week, and tumor sizes were measured with a glide caliper. Tumor volume was calculated using the formula (length [mm] × width [mm]2) ÷ 2. (B) Gross appearances of mice representative of each injection group (i), appearances of tumor or normal skin (Hut78 TOX-sh) from representative mice (ii), H&E staining of tissue sections from corresponding tumors or normal skin (iii), and immunohistochemistry staining of human CD3 from corresponding tumors or normal skin (iv). Bars represent 1 cm (Bi-ii), 200 μm (Biii-iv), and 25 μm (Biii-iv insets).
TOX inhibition impairs the tumor-forming ability of CTCL cells in vivo. (A) Tumor volume comparison between Hut78 and HH mice injected with 1 million TOX-sh cells (n = 12) or control (CTR) cells (n = 6). Mice were monitored for local tumor formation 3 times a week, and tumor sizes were measured with a glide caliper. Tumor volume was calculated using the formula (length [mm] × width [mm]2) ÷ 2. (B) Gross appearances of mice representative of each injection group (i), appearances of tumor or normal skin (Hut78 TOX-sh) from representative mice (ii), H&E staining of tissue sections from corresponding tumors or normal skin (iii), and immunohistochemistry staining of human CD3 from corresponding tumors or normal skin (iv). Bars represent 1 cm (Bi-ii), 200 μm (Biii-iv), and 25 μm (Biii-iv insets).
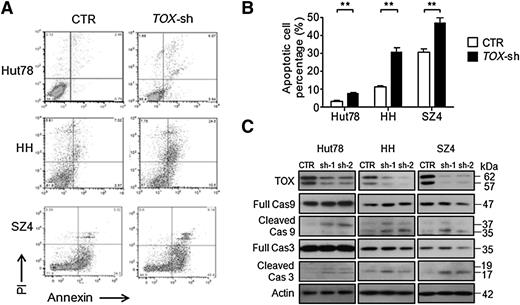
TOX suppression induces apoptosis and caspase activation in CTCL cells
To elucidate the mechanisms through which TOX silencing exerts its antiproliferative effect, we assessed whether the apoptosis machinery was affected. With TOX knockdown, transduced CTCL cells had increased apoptotic cells, demonstrated by annexin V positivity (Figure 5A-B). On the molecular level, TOX knockdown led to activation of caspase 9 and caspase 3, which are involved in apoptosis initiation and execution (Figure 5C). The activation was more obvious in HH and SZ4 cells, consistent with the more dramatic apoptosis sensitization phenotype.
TOX suppression leads to increased apoptosis and caspase activation in CTCL cells. (A) FACS analysis of apoptotic (annexin V–positive) cell population in TOX-sh Hut78, HH, and SZ4 cells compared to control (CTR) cells. (B) Percentages of apoptotic cells in TOX-sh Hut78, HH, and SZ4 cells compared to CTR cells. **P < .01 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 4 independent experiments. (C) Comparison of caspase 9 and caspase 3 levels (both full-length and cleaved) in TOX-sh and CTR cells. PI, propidium iodide.
TOX suppression leads to increased apoptosis and caspase activation in CTCL cells. (A) FACS analysis of apoptotic (annexin V–positive) cell population in TOX-sh Hut78, HH, and SZ4 cells compared to control (CTR) cells. (B) Percentages of apoptotic cells in TOX-sh Hut78, HH, and SZ4 cells compared to CTR cells. **P < .01 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 4 independent experiments. (C) Comparison of caspase 9 and caspase 3 levels (both full-length and cleaved) in TOX-sh and CTR cells. PI, propidium iodide.
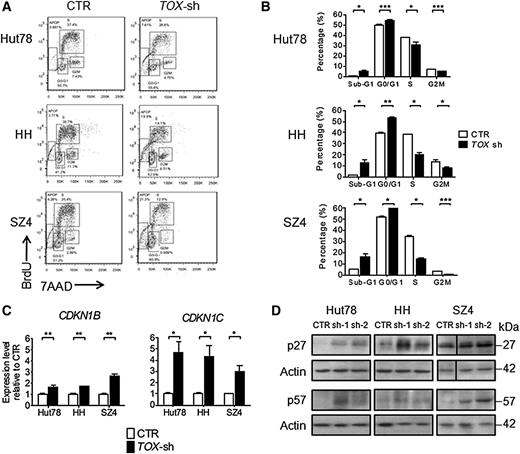
TOX suppression arrests cell cycle and restores key cell cycle inhibitors in CTCL cells
We next assessed cell cycle progression, another key process governing cellular growth. Blocking of TOX markedly affected cell cycle control at both G1-S and S-G2M transitions in all 3 cell lines (Figure 6A-B). In light of this observation, we asked how TOX silencing affected the cell cycle regulatory machinery by evaluating the expression changes of several critical cell cycle regulators, including CDK2, CDK4, CDKN1A, CDKN1B, and CNKN1C. TOX suppression increased the expression of CDKN1B and CDKN1C in all 3 cell lines (Figure 6C) without consistently changing the levels of CDK2, CDK4, and CDKN1A (data not shown). This finding was confirmed by western blot analysis, which showed that p27 protein (encoded by CDKN1B) and p57 protein (encoded by CDKN1C) were also upregulated after TOX knockdown (Figure 6D).
TOX suppression leads to cell cycle arrest and elevated cell cycle repressors. (A) FACS analysis of cell proliferation by BrdU incorporation in TOX-sh Hut78, HH, and SZ4 cells compared to control (CTR) cells. For BrdU incorporation assay, transduced Hut78 cells were pulsed with 10 μM BrdU for 45 minutes, whereas transduced HH and SZ4 cells were pulsed for 2 hours. (B) Cell cycle distribution in TOX-sh cells compared to CTR cells. (C) CDKN1B and CDKN1C transcript levels in TOX-sh and CTR. qRT-PCR was performed using primers specific for CDKN1B, CDKN1C, and GAPDH mRNA. The level of CDKN1B and CDKN1C mRNA was normalized to that of GAPDH and is depicted as the fold change compared to CTR cells. *P < .05, **P < .01, and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 3 independent experiments. (D) p27 and p57 protein levels in TOX-sh and CTR cells.
TOX suppression leads to cell cycle arrest and elevated cell cycle repressors. (A) FACS analysis of cell proliferation by BrdU incorporation in TOX-sh Hut78, HH, and SZ4 cells compared to control (CTR) cells. For BrdU incorporation assay, transduced Hut78 cells were pulsed with 10 μM BrdU for 45 minutes, whereas transduced HH and SZ4 cells were pulsed for 2 hours. (B) Cell cycle distribution in TOX-sh cells compared to CTR cells. (C) CDKN1B and CDKN1C transcript levels in TOX-sh and CTR. qRT-PCR was performed using primers specific for CDKN1B, CDKN1C, and GAPDH mRNA. The level of CDKN1B and CDKN1C mRNA was normalized to that of GAPDH and is depicted as the fold change compared to CTR cells. *P < .05, **P < .01, and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 3 independent experiments. (D) p27 and p57 protein levels in TOX-sh and CTR cells.
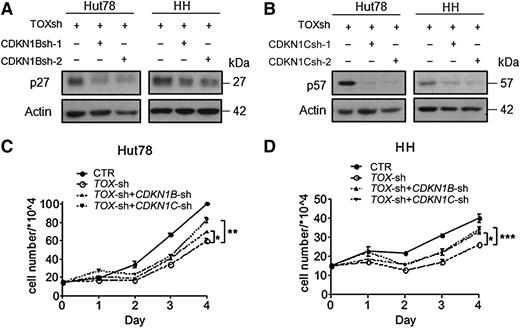
P27 and p57 mediate the growth inhibitory effect of TOX suppression
To examine whether the enhanced p27 and p57 proteins are responsible for the growth arrest in CTCL cells on TOX suppression, we performed additional stable knockdown of CDKN1B and CDKN1C in the TOX-suppressed CTCL cells. Due to the cytotoxicity of a second virus infection in SZ4 TOX-sh cells, which was rapidly fatal, we were only able to obtain double knockdown in Hut78 and HH cells. Nevertheless, stable knockdown of either CDKN1B or CDKN1C in Hut78 TOX-sh and HH TOX-sh cells (Figure 7A-B) effectively reversed the proliferation suppression by TOX knockdown (Figure 7C-D). These results indicate that p27 and p57 are responsible, at least in part, for the growth inhibitory effect of TOX suppression in CTCL cells.
Knockdown of CDKN1B or CDKN1C reverses growth inhibition of TOX-sh cells. (A) CDKN1B knockdown by 2 shRNA (sh-1 and sh-2) in addition to TOX knockdown in Hut78 and HH cells. Transduced cells were selected by hygromycin (750 μg/mL for Hut78 and 200 μg/mL for HH) for 7 days before the protein lysates were probed with antibodies against p27 and actin proteins. (B) CDKN1C knockdown in addition to TOX knockdown in Hut78 and HH cells. Transduced cells were selected by hygromycin for 7 days before western blot analysis was performed to examine p57 protein levels. (C) Cosilencing of TOX with CDKN1B or CDKN1C led to increased proliferative rate in Hut78 cells. A total of 1.5 × 105 cells were cultured in 2 mL of full RPMI media for 4 days, and viable cells were determined each day by the trypan blue exclusion method. (D) Cosilencing of TOX with CDKN1B or CDKN1C led to increased proliferative rate in HH cells. Experiments were conducted in the same way as for Hut78 cells. *P < .05, **P < .01, and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 3 independent experiments.
Knockdown of CDKN1B or CDKN1C reverses growth inhibition of TOX-sh cells. (A) CDKN1B knockdown by 2 shRNA (sh-1 and sh-2) in addition to TOX knockdown in Hut78 and HH cells. Transduced cells were selected by hygromycin (750 μg/mL for Hut78 and 200 μg/mL for HH) for 7 days before the protein lysates were probed with antibodies against p27 and actin proteins. (B) CDKN1C knockdown in addition to TOX knockdown in Hut78 and HH cells. Transduced cells were selected by hygromycin for 7 days before western blot analysis was performed to examine p57 protein levels. (C) Cosilencing of TOX with CDKN1B or CDKN1C led to increased proliferative rate in Hut78 cells. A total of 1.5 × 105 cells were cultured in 2 mL of full RPMI media for 4 days, and viable cells were determined each day by the trypan blue exclusion method. (D) Cosilencing of TOX with CDKN1B or CDKN1C led to increased proliferative rate in HH cells. Experiments were conducted in the same way as for Hut78 cells. *P < .05, **P < .01, and ***P < .001 by 2-tailed Student t test with Welch correction. Error bars indicate standard error of the mean. Data depicted are representative of at least 3 independent experiments.
Discussion
TOX is a member of the evolutionarily conserved HMG box family and a key regulatory nuclear protein in the development of CD4+ T cells, natural killer cells, and lymphoid tissue inducer cells.13-15,30,31 The roles of TOX in immune system development are relatively well-characterized since its discovery13 ; however, its role in human cancer has not been reported. On the basis of our findings, TOX has emerged as a key player, when expressed ectopically, in driving malignant cell transformation in CTCL.
The molecular pathogenesis of SS has been a topic of active investigation in the last decade. However, the molecular nature of SS cells has only started to be understood. Aberrations in signal transducers and transcription factors have been reported in SS studies, such as Jun B, JunD, TGFBR2, STAT3, STAT4, CDKN1C, CTLA-4, GATA3, AHI-1, SATB1, PDCD10, and NOTCH1.4,16,17,32-36 Several of these dysregulated genes (TGFBR2, GATA3, STAT3, SATB1, and NOTCH1) are involved in the signal cascades governing T-cell development.37-41 Our recent observation of aberrant TOX upregulation in MF, subsequently confirmed by McGirt et al,10 prompted us to ask whether TOX is also upregulated in SS, the advanced CTCL, and whether TOX contributes to the development of CTCL.
Not surprisingly, TOX was also highly expressed on mRNA and protein levels in primary Sézary cells, as well as in several patient-derived CTCL cell lines. Although TOX is rapidly induced by TCR signaling in the developing thymocytes,13 we observed a drastic reduction of TOX expression in normal mature CD4+ T cells; therefore, it is unlikely that the aberrant TOX expression in CTCL was due to continuous TCR activation. Furthermore, we detected higher TOX levels in Sézary cells with positive TCR clonality compared to those without TCR clonality. Because TCR clonality, when present, is closely correlated with worse clinical outcome of SS patients,42,43 we wondered whether TOX transcript levels are linked to disease outcome. Indeed, higher TOX expression levels in SS patients correlated with increased risk of SS-related death, in line with our previous report in a cohort of MF patients (n = 59), which found that high TOX mRNA levels correlated with increased risks of disease progression and disease-specific mortality.44 Together, these findings pointed to the possibility that TOX contributed to the development of CTCL.
This possibility was tested and confirmed in the current study using cultured CTCL cells and xenograft mouse models. In 3 CTCL cell lines, TOX suppression by lentivirus-mediated shRNA gene silencing markedly normalized the CTCL cells’ resistance to apoptosis and abnormal cell cycle progression, the 2 best-characterized features of CTCL cells.5-8 In agreement with in vitro data, subcutaneous injection of CTCL cells into NSG mice confirmed this proliferative disadvantage, in that the ability of TOX-suppressed CTCL cells to induce local tumors was significantly impaired or even abolished. In aggregate, these findings demonstrate the biological importance of sustained TOX activation to the leukemic activities of CTCL cells.
We next explored the mechanism underlying the transforming properties of TOX by identifying its downstream molecular partners. The dramatic cell cycle arrest phenotype led us to examine how the cell cycle control machinery was affected by TOX suppression. We found that 2 key cell cycle regulators, CDKN1B and CDKN1C, were increased on TOX suppression. We further confirmed that p27 protein (encoded by CDKN1B) and p57 protein (encoded by CDKN1C) were also increased in TOX-suppressed cells.
CDKN1B and CDKN1C were frequently lost in many lymphoid malignancies, including SS.18,45,46 Moreover, a recent study reported that CDKN1C deficiency was associated with poor outcome in CTCL patients.47 Intriguingly, in addition to their role in cell cycle control, a dual role in apoptosis has been documented for both p27 and p57.48-51 Therefore, they appear to be promising mediators of the dual effects of TOX on apoptosis and cell cycling. Indeed, additional stable knockdown of CDKN1B or CDKN1C increased growth of CTCL cells in the face of loss of TOX, thus reversing the effect of TOX knockdown. These data suggest that the leukemic activities of TOX in CTCL, at least in part, rely on repressing the transcription of CDKN1B and CDKN1C. Given that silencing of either gene was not able to fully restore uncontrolled cell growth, it is likely that additional partners are involved in mediating the functions of TOX.
CDKN1B transcription can be activated by the binding of several transcription factors, although the changes of CDKN1B are largely regulated posttranslationally.52 CDKN1C is an imprinted gene rich in CpG islands near its putative transcription start site. Its transcription is under the control of numerous pathways, with epigenetic control and microRNA regulation being the best characterized.53-57 Several mechanisms are possible as to how TOX regulates CDKN1B and CDKN1C, including direct binding to their promoters or modulating local chromatin structure and formation of multiprotein complexes, a mechanism generally employed by HMG box proteins.13 Further experiments in the future may help clarify the precise mechanism of how TOX regulates p27 and p57.
In conclusion, we provide strong evidence that TOX is highly expressed in SS and exerts transforming activities in CTCL. On TOX silencing, growth of CTCL cells was markedly slowed or halted in vitro and in vivo because of cell cycle arrest and apoptosis sensitization. This effect is partially mediated by 2 key CDK inhibitors, CDKN1B and CDKN1C. Correction of TOX, CDKN1B/CDKN1C, or both may therefore be an appropriate therapeutic approach to harness the uncontrolled tumor growth.
Presented in part in abstract form at the 73rd annual meeting of the Society for Investigative Dermatology, Albuquerque, NM, May 7-10, 2014.1
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Wang, R. Yu, and G. Zhang for their excellent technical assistance. Y.H. is a PhD candidate at the University of British Columbia and this work is submitted in partial fulfillment of the requirement for a PhD.
This work was supported by grants from the Canadian Institutes of Health Research (Y.Z. and X.J.) and the Canadian Dermatology Foundation (Y.Z.). Y.H. is a recipient of a Canadian Institutes of Health Research Skin Research Training Center Scholarship and a Vanier Canada Graduate Scholarship.
Authorship
Contribution: Y.H. and M.-W.S. designed and performed the experiments and analyzed the data; X.J. and Y.Z. developed the concept of the study and designed and supervised the experiments; Y.H. and Y.Z. wrote the manuscript; and M.-W.S. and X.J. commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Youwen Zhou, Department of Dermatology and Skin Sciences, University of British Columbia, 835 West 10th Ave, Vancouver, BC, Canada V5Z 4E8; e-mail: youwen.zhou@ubc.ca; and Xiaoyan Jiang, Terry Fox Laboratory, British Columbia Cancer Agency, 675 West 10th Ave, Vancouver, BC, Canada V5Z 1L3; e-mail: xjiang@bccrc.ca.

![Figure 1. TOX mRNA levels are increased in peripheral blood CD4+ T cells from SS patients and correlate with disease-specific mortality. (A) TOX mRNA levels in peripheral blood CD4+ T cells from SS patients (n = 12), HPs (n = 9), and BID patients (psoriasis [PSO], n = 8; rosacea [ROS], n = 5; vitiligo [VT], n = 5). In each sample, RNA was extracted as described in the “Methods and materials” section. qRT-PCR was performed using primers specific for TOX and GAPDH mRNA. The level of TOX mRNA was normalized to that of GAPDH so that the levels shown represent copies of TOX mRNA per 1000 copies of GAPDH mRNA. (B) TOX mRNA levels in CD4+ T cells from SS patients with clonality (n = 7) vs without clonality (n = 5). (C) Receiver operating characteristic curve of TOX mRNA levels in distinguishing SS from non-SS. (D) High TOX mRNA levels in peripheral CD4+ T cells correlated with a higher disease-specific mortality. TOX high and TOX low groups were defined by the expression levels higher and lower than 129.9, respectively. AUC, area under the curve.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/9/10.1182_blood-2014-05-571778/4/m_1435f1.jpeg?Expires=1769147402&Signature=epVBczutUw-lTF6BW7XSUHGCR0ycYTXD3cXkFgm4ajCnpcVZqoelbMsR1hKIzYcymMCprqqYHVgsf30gCIOdT~3Y2IB1JbyHG1pvbBjFROwyGMDsI~QQigUvChyH4NdPG0lq8be7G3QBzzlmvTmIYmPZ3VqsK9qerA5tFUuSrxpr4ZsLxG1-6eVRQ83Py8Na~55RRCT0avuQVA2-u-O43uYZ~L2kb8usHPgWyHoefiATbUlDdpHQ-M2x9qqWNpWmBA64g8AxKlyePoTvFnyMs53EcT09DR2yMHEAwiK0FMLBv7neza04fZKX6azNWZLH9JxMK7iixrCAXpmSGkNXLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. TOX inhibition impairs the tumor-forming ability of CTCL cells in vivo. (A) Tumor volume comparison between Hut78 and HH mice injected with 1 million TOX-sh cells (n = 12) or control (CTR) cells (n = 6). Mice were monitored for local tumor formation 3 times a week, and tumor sizes were measured with a glide caliper. Tumor volume was calculated using the formula (length [mm] × width [mm]2) ÷ 2. (B) Gross appearances of mice representative of each injection group (i), appearances of tumor or normal skin (Hut78 TOX-sh) from representative mice (ii), H&E staining of tissue sections from corresponding tumors or normal skin (iii), and immunohistochemistry staining of human CD3 from corresponding tumors or normal skin (iv). Bars represent 1 cm (Bi-ii), 200 μm (Biii-iv), and 25 μm (Biii-iv insets).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/9/10.1182_blood-2014-05-571778/4/m_1435f4.jpeg?Expires=1769147402&Signature=3Skek~AJ8ymQZHNHRtY0XjKqRGMHmREq4FtCRv6CfTxCrQg6RgTZZbv4JdkQn~Q3EiUI3a-gPwrdLB8Zu57473tf~1ajID0-xDaPdPb9iys6se8Y5cAHLhlvjT6Z3EUBfQaY17-w9H7jQmTaidD5p-iy6KfAT5SRvxoCtlreRn~XJJ-K5Os7JXQ9UkhKD2ii6abIFHAJt2mjcEi1d-F-hmfZmt3T63HNge5vQgg23imPH7JpQsXfHFDeJOpkhO3En0QW4s0mxo4QZozubT9IybfAF1eruGbOv0vWbOFm1dXUUA~M72E5SUoxsh8nqyzUSCxgzNDl6lrYRXZfoVyq3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)