Key Points
SRL and PDN often induce disease stabilization or objective responses in patients with ECD.
The phosphorylated forms of mTOR and of its downstream kinase p70S6K are strongly expressed in infiltrating histiocytes.
Abstract
Erdheim-Chester disease (ECD) is a rare non-Langerhans cell histiocytosis, to whose pathogenesis neoplastic and immune-mediated mechanisms contribute. Mammalian target of rapamycin (mTOR)-inhibitors have antiproliferative and immunosuppressive properties. We tested in this study, the efficacy and safety of the mTOR-inhibitor sirolimus (SRL) plus prednisone (PDN) in patients with ECD. PDN was given initially at 0.75 mg/kg per day, tapered to 5 to 2.5 mg per day by month 6. Target SRL blood levels were 8 to 12 ng/mL. Treatment was continued for at least 24 months in patients who showed disease stabilization or improvement. Ten patients were enrolled; 8 achieved stable disease or objective responses, whereas 2 had disease progression. Responses were mainly observed at the following sites: retroperitoneum in 5/8 patients (62.5%), cardiovascular in 3/4 (75%), bone in 3/9 (33.3%), and central nervous system (CNS) in 1/3 (33.3%). The median follow-up was 29 months (interquartile range, 16.5-74.5); 2 patients died of progressive CNS disease and small-cell lung cancer, respectively. Treatment-related toxicity was mild. Using immunohistochemistry and immunofluorescence on ECD biopsies, we detected expression in foamy histiocytes of the phosphorylated forms of mTOR and of its downstream kinase p70S6K, which indicated mTOR pathway activation. In conclusion, SRL and PDN often induce objective responses or disease stabilization and may represent a valid treatment of ECD. The trial is registered at the Australia-New Zealand Clinical Trial Registry as #ACTRN12613001321730.
Introduction
Erdheim-Chester disease (ECD) is an extremely rare and often life-threatening form of non-Langerhans cell histiocytosis, characterized by tissue infiltration of CD68+ CD1a− “foamy” histiocytes. To date, only ∼550 cases of ECD have been reported in the literature.1 The most frequent manifestations of ECD include symmetric long bone osteosclerosis, retroperitoneal infiltration (peri-aortic and peri-renal fibrosis), and cardiovascular lesions (pericarditis, myocardial infiltration, and peri-aortic sheathing); the central nervous system (CNS), the retro-orbital space, the skin, and various endocrine axes are also frequently affected.2,3
The pathogenesis of ECD is still unclear. Initial studies investigating the clonal nature of the infiltrating histiocytes yielded conflicting results4,5 ; however, the recent observation that ∼50% of ECD cases harbor the V600E mutation of the BRAF proto-oncogene,6 together with other reports showing NRAS mutations,7,8 indicate that ECD can be clonally driven. Immune-mediated mechanisms are also of pathogenetic importance. ECD histiocytes show intense expression of chemokines and their receptors and produce pro-inflammatory cytokines9,10 ; the serum cytokine profile demonstrates T helper-1 polarization, with upregulation of interleukin (IL)-12, interferon (IFN)-γ inducible protein-10, and monocyte chemotactic protein-1.11
Different therapeutic approaches have been used for ECD. IFN-α is considered the first-line therapy and is thought to induce immune-mediated histiocyte killing and terminal differentiation of immature histiocytes. However, IFN-α is often ineffective and poorly tolerated.3 The BRAFV600E inhibitor vemurafenib proved markedly effective in BRAFV600E-mutated cases,12,13 but the BRAFV600E mutation is either absent or not detected in ∼50% of ECD patients,14 therefore therapeutic alternatives are needed. Other treatments include antineoplastic drugs (eg, cladribine and imatinib mesylate)15 and immunosuppressive agents such as IL-1–receptor- and tumor necrosis factor-α–blocking antibodies,16,17 but these drugs have only been tested in single cases or small case series.
The mammalian target of rapamycin (mTOR) integrates extracellular and intracellular signals to regulate cell growth, proliferation and apoptosis, and several metabolic processes such as protein and lipid synthesis. mTOR also modulates immune responses, as it promotes differentiation and activation of B cells, T cells, and antigen-presenting cells.18 Aberrant mTOR activation is found in neoplastic and inflammatory conditions, so mTOR inhibitors (given their antiproliferative and immunosuppressive properties) are now used in different settings, including malignancies19 and the prevention of allograft rejection.20 Interestingly, a recent study showed that some (although only ∼11%) ECD patients harbor PIK3CA mutations, which lead to mTOR pathway activation.14
On the basis of the above considerations, particularly the dual neoplastic-inflammatory nature of ECD, we used the mTOR inhibitor sirolimus (SRL) in combination with prednisone (PDN) for patients with multisystemic ECD. We report here the results of our trial, which tested the efficacy and safety of this approach in 10 consecutive ECD patients, and also provide preliminary evidence of mTOR pathway activation in ECD lesions.
Methods
Patients
Patient #1, treated empirically with SRL+PDN from June 2005, represented our index case. Encouraged by his clinical response, which persisted after 2 years of treatment, we designed this open-label trial. We enrolled all patients meeting the eligibility criteria, referred to the Nephrology Unit of Parma University Hospital between December 2007 and June 2013. Key inclusion criteria were a diagnosis of multisystemic ECD based on Veyssier-Belot criteria21 and an age of 18 to 75 years. We enrolled both newly diagnosed, untreated patients, as well as those patients who proved refractory to previous treatments. Key exclusion criteria were concurrent neoplasms or other forms of histiocytosis, serious infections, uncontrolled diabetes, estimated glomerular filtration rate <30 mL/min, proteinuria >1 g/24 h, and recent major surgery.
The patients signed an informed consent form. The study was performed in accordance with the Declaration of Helsinki and was approved by the Parma Ethical Committee.
Treatment protocol and assessment of response
At study entry, the patients underwent physical examination and routine laboratory tests, including C-reactive protein levels and endocrine tests (thyrotropin, FT3, FT4, prolactin, luteinizing hormone, follicle-stimulating hormone, testosterone, estradiol, cortisol, and adrenocorticotropic hormone). We also performed long-bone radiographs, 99Tc-bone scintigraphy, high-resolution chest computed tomography (CT), contrast-enhanced abdominal CT or magnetic resonance imaging (MRI), brain MRI, and echocardiography. As 18F-fluorodeoxyglucose (18F-FDG)-positron emission tomography (PET) became available for this indication at Parma University Hospital in 2009, it was performed in only 7 patients. Likewise, cardiac MRI became routinely available at our center in 2010, thus it was performed in only 5 patients.
All patients received PDN and SRL. PDN was given orally at a single daily dose of 0.75 mg/kg per day for month 1, 0.5 mg/kg per day for month 2, 0.25 mg/kg per day for months 3 to 4, and 0.125 mg/kg per day for months 5 to 6. After month 6, it was tapered to a maintenance dose of 2.5 to 5 mg per day. SRL was given orally at an initial dose of 2 mg/day (single daily dose), and subsequently titrated to reach blood levels of 8 to 12 ng/mL; this blood level range was chosen based on the experience accumulated in solid organ transplantation and in renal angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis.20,22 Blood SRL levels were checked fortnightly during the first 2 months and subsequently every 1 to 2 months. The target SRL range remained unchanged throughout the study. Proton-pump inhibitors, calcium, vitamin D, and statins were also routinely prescribed.
Treatment was conducted until the first assessment of response (month 6); if disease stabilization or remission was achieved, treatment was continued until month 24. In case of disease progression, it was withdrawn and choice of the alternative therapy was left at the discretion of the treating clinician. After month 24, the physician was free to continue or withdraw treatment based on patient’s status and treatment tolerability.
Follow-up visits, which included clinical and laboratory examinations, were done every 1 to 2 months during the first year and every 3 to 6 months thereafter. Complete disease re-staging was performed at month 6, 12, and every 12 months thereafter; in addition, patients were scanned as soon as new or worsening symptoms appeared. Responses were assessed clinically and radiologically, using the same imaging modalities performed at baseline. Whole-body PET-CT was repeated only in patients with pathologic uptake at baseline.
Objective responses were defined on CT or MRI findings as complete or partial responses (PRs), stable disease (SD), and progressive disease (PD), generally following the RECIST criteria (version 1.0).23 Briefly, complete responses (CRs) indicated the disappearance of a given lesion, and PRs a decline of at least 30% in the sum of the longest lesion diameters; SD was defined as neither PR nor PD, and PD as an increase of at least 20% in the sum of lesion diameters or appearance of new lesions. For specific lesions such as peri-aortic fibrosis, the maximal thickness of the tissue was recorded at infra-renal aorta and common iliac artery levels,24 and the average of these measures was calculated. For nonmeasurable lesions (eg, bone infiltration), we followed the RECIST 1.0 grading as CR, incomplete response/SD, and PD.
On 18F-FDG-PET, the response was graded on the changes of the maximum standardized uptake value (SUVmax), generally following the PERCIST criteria25 ; patients were thus categorized as having complete or partial metabolic responses (PMRs) (complete resolution or decline of at least 30% of 18F-FDG uptake, respectively), stable metabolic disease (neither PMR nor progressive metabolic disease), or progressive metabolic disease (increase of at least 30% in target lesion activity or appearance of new lesions).
Treatment-related toxicity was assessed at each visit by a checklist of standardized items, measurement of blood pressure and body weight, and routine laboratory tests. Adverse events (AEs) were graded following the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.26
Biopsy examination and analysis of mTOR pathway activation
Tissue biopsies were centrally reviewed by a pathologist with expertise in soft-tissue tumors (D.C.); routine stainings and immunohistochemical analysis of CD68 and CD1a expression were performed. BRAFV600E mutation was assessed on the available biopsies. Details on histologic and immunohistochemical analyses and BRAFV600E testing are reported in supplemental Methods on the Blood Web site. Where sufficient biopsy tissue was available, we also searched for recurrent mutations in the NRAS, KRAS, and PIK3CA genes (supplemental Methods).
To assess whether mTOR pathway is active in ECD lesions, and in particular in ECD histiocytes, we assessed the expression of phospho-p70S6K, phospho-mTOR, and CD68 using immunohistochemistry (IHC) and immunofluorescence (supplemental Methods).
Statistical analysis
Data are presented as median (interquartile range [IQR]); survival was estimated using the Kaplan–Meier method. All analyses were done using SPSS version 20.0 (SPSS Inc., Chicago, IL).
Results
After our index case (patient #1), we screened 11 consecutive patients, of whom 2 did not meet the eligibility criteria; the remaining 9 were enrolled. The results presented here refer to the whole series of 10 patients, including the index case; patient enrollment and follow-up is schematically described in the flow-chart (supplemental Figure 1). All but 1 patient (patient #6) had biopsy-proven ECD; in the nonbiopsy proven case, the diagnosis was based on typical bone scintigraphy and long-bone radiograph findings, together with the presence of CNS lesions compatible with ECD. Three patients were refractory to previous treatments, whereas 7 were newly diagnosed and untreated. The main patient characteristics are described in Table 1.27 Long-bone involvement was found in 9 patients, although only 4 complained of bone pain. Retroperitoneal involvement was also common: most patients showed peri-aortic, peri-renal, and peri-ureteral fibrosis causing hydronephrosis, which required ureteral double-J stent placement in 4 cases. Notably, in 4 patients, peri-aortic fibrosis also involved the mesentery; 2 of these patients developed repeat bowel obstructions requiring surgery. Cardiac and thoracic aorta involvement was found in 4 cases, although it must be acknowledged that only 5 patients were studied using cardiac MRI (the remaining with echocardiography); 2 patients had pericarditis with massive pericardial effusion, with 1 patient developing tamponade. CNS involvement was detected in 3 patients: 1 had a large hemispheric mass (that was surgically excised) and small cortical and brainstem nodules, 1 had diffuse cortical-subcortical nodules, and 1 a very severe involvement of the brainstem and the cerebellar peduncles together with supra-tentorial lesions. Interstitial lung disease was radiologically detected in 3 patients, none of whom was symptomatic. Other manifestations, including endocrine abnormalities, skin lesions, exophthalmos, and soft-tissue masses were also found (Table 1). Finally, most patients had systemic symptoms: in particular, 5 patients (patients #1, 2, 3, 8, and 10) complained of fatigue, 2 (patients #3 and #8) had fever, 2 had weight loss (patients #6 and #8), and 1 (patient #8) developed severe cachexia, aggravated by intestinal obstruction episodes.
Main characteristics of the enrolled patients at study entry
| . | . | . | Involved sites and/or main disease manifestations . | Mutations . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # . | Age (y), gender . | Previous therapies . | Bone . | Cardiovascular system . | Retroperitoneum . | Endocrine system . | CNS . | Lung . | Other . | BRAF . | NRAS . | KRAS . | PIK3CA . |
| 1 | 68, M | PDN + CYC | Long bones (lower limbs) | Coated aorta | Peri-renal and peri-aortic fibrosis, bilateral HDN, and mesenteric fibrosis with repeat bowel occlusion | — | — | — | Bilateral leg edema and systemic symptoms | Negative (by IHC) | N/A | N/A | N/A |
| 2 | 47, M | — | Long bones (upper and lower limbs) | Pericarditis with tamponade, atrial mass, and coated aorta | Peri-renal and peri-aortic fibrosis | Hyper-prolactinemia (pituitary involvement on MRI) | — | — | Skin nodules | V600E | WT | WT | WT |
| 3 | 37, F | — | Long bones (upper and lower limbs) | — | Peri-aortic and mesenteric fibrosis | Pituitary enlargement (on MRI) | Supratentorial parenchymal nodules | — | Skin nodule | WT | WT | WT | WT |
| 4 | 44, M | IFN-α | Long bones (lower limbs), skull and facial bones, and left clavicle | — | Peri-renal and peri-aortic fibrosis, and bilateral HDN | Diabetes insipidus, (central) hypothyroidism and hypogonadism; and MRI evidence of pituitary stalk involvement | — | Interstitial lung disease | — | N/A | N/A | N/A | N/A |
| 5 | 69, M | — | Long bones (lower limbs), and facial bones | — | Peri-aortic and peri-renal fibrosis, and left-sided HDN | Peripheral hypogonadism | — | — | — | V600E | WT | WT | WT |
| 6 | 75, M | — | Long bones (lower limbs), facial bones, right clavicle, ribs, and spine | — | — | — | Diffuse involvement of midbrain, pons, cerebellar peduncles, supra-tentorial nodules | Interstitial lung disease | — | N/A | N/A | N/A | N/A |
| 7 | 43, M | — | Long bones (lower limbs) | — | — | — | Cortical and pons nodules, and bulky emispheric mass in the parieto-occipital left lobes | — | Xanthelasma, soft-tissue masses (para-vertebral, Achille's tendons, nose, and right thigh) | NA | NA | NA | NA |
| 8 | 42, F | PDN + colchicine | Long bones (upper and lower limbs) and facial bones | Coated aorta, myocardial infiltration with coronary involvement, and atrial mass | Peri-aortic and peri-renal fibrosis, bilateral HDN, and mesenteric fibrosis with ascites | (Peripheral) hypogonadism | — | Interstitial lung disease | Exophthalmos, xanthelasmas, digital ischemia, diffuse lymph-node enlargement, and bilateral leg edema | V600E | WT | WT | WT |
| 9 | 71, M | — | — | — | Peri-renal fibrosis | — | — | — | Exophthalmos | N/A | N/A | N/A | N/A |
| 10 | 46, M | — | Long bones (upper and lower limbs) and facial bones | Pericarditis with massive pericardial effusion, myocardial infiltration with coronary involvement, and coated aorta | Peri-aortic and peri-renal fibrosis, bilateral HDN, and mesenteric fibrosis | (Peripheral) hypogonadism | — | — | Exophthalmos | WT | WT | WT | WT |
| . | . | . | Involved sites and/or main disease manifestations . | Mutations . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # . | Age (y), gender . | Previous therapies . | Bone . | Cardiovascular system . | Retroperitoneum . | Endocrine system . | CNS . | Lung . | Other . | BRAF . | NRAS . | KRAS . | PIK3CA . |
| 1 | 68, M | PDN + CYC | Long bones (lower limbs) | Coated aorta | Peri-renal and peri-aortic fibrosis, bilateral HDN, and mesenteric fibrosis with repeat bowel occlusion | — | — | — | Bilateral leg edema and systemic symptoms | Negative (by IHC) | N/A | N/A | N/A |
| 2 | 47, M | — | Long bones (upper and lower limbs) | Pericarditis with tamponade, atrial mass, and coated aorta | Peri-renal and peri-aortic fibrosis | Hyper-prolactinemia (pituitary involvement on MRI) | — | — | Skin nodules | V600E | WT | WT | WT |
| 3 | 37, F | — | Long bones (upper and lower limbs) | — | Peri-aortic and mesenteric fibrosis | Pituitary enlargement (on MRI) | Supratentorial parenchymal nodules | — | Skin nodule | WT | WT | WT | WT |
| 4 | 44, M | IFN-α | Long bones (lower limbs), skull and facial bones, and left clavicle | — | Peri-renal and peri-aortic fibrosis, and bilateral HDN | Diabetes insipidus, (central) hypothyroidism and hypogonadism; and MRI evidence of pituitary stalk involvement | — | Interstitial lung disease | — | N/A | N/A | N/A | N/A |
| 5 | 69, M | — | Long bones (lower limbs), and facial bones | — | Peri-aortic and peri-renal fibrosis, and left-sided HDN | Peripheral hypogonadism | — | — | — | V600E | WT | WT | WT |
| 6 | 75, M | — | Long bones (lower limbs), facial bones, right clavicle, ribs, and spine | — | — | — | Diffuse involvement of midbrain, pons, cerebellar peduncles, supra-tentorial nodules | Interstitial lung disease | — | N/A | N/A | N/A | N/A |
| 7 | 43, M | — | Long bones (lower limbs) | — | — | — | Cortical and pons nodules, and bulky emispheric mass in the parieto-occipital left lobes | — | Xanthelasma, soft-tissue masses (para-vertebral, Achille's tendons, nose, and right thigh) | NA | NA | NA | NA |
| 8 | 42, F | PDN + colchicine | Long bones (upper and lower limbs) and facial bones | Coated aorta, myocardial infiltration with coronary involvement, and atrial mass | Peri-aortic and peri-renal fibrosis, bilateral HDN, and mesenteric fibrosis with ascites | (Peripheral) hypogonadism | — | Interstitial lung disease | Exophthalmos, xanthelasmas, digital ischemia, diffuse lymph-node enlargement, and bilateral leg edema | V600E | WT | WT | WT |
| 9 | 71, M | — | — | — | Peri-renal fibrosis | — | — | — | Exophthalmos | N/A | N/A | N/A | N/A |
| 10 | 46, M | — | Long bones (upper and lower limbs) and facial bones | Pericarditis with massive pericardial effusion, myocardial infiltration with coronary involvement, and coated aorta | Peri-aortic and peri-renal fibrosis, bilateral HDN, and mesenteric fibrosis | (Peripheral) hypogonadism | — | — | Exophthalmos | WT | WT | WT | WT |
Unless stated otherwise, the mutations were assessed using molecular techniques (see text for details); only in 1 patient (patient #1) did we assess it by IHC only.
“Coated aorta” indicates perivascular infiltration of the thoraco-abdominal aorta (Diamond et al27 ).
CYC, cyclophosphamide; HDN, hydronephrosis; N/A, biopsy not available (or not enough to allow sufficient nucleic acid amplification); V600E, mutation V600E of BRAF; WT, wild-type.
The BRAFV600E mutation was detected in 3/6 patients in whom it was assessed; no mutations in NRAS, KRAS, or PKI3CA were detected in the tested patients (Table 1).
All patients received SRL and PDN following the treatment schedule, with no significant protocol deviations. Objective responses are reported in Table 2. Eight patients showed either disease stabilization or partial/CRs, whereas 2 patients had disease progression; of these, patient #6 had rapidly progressive CNS lesions, refused to take IFN-α and switched to IM methotrexate but resumed SRL after 1 month because of poor methotrexate tolerability. He eventually died after 11 months of treatment. The second patient with PD (patient #8) had SD at months 6 to 12, but later progressed at cardiac and retroperitoneal levels; she switched to vemurafenib (at month 25) as she was found to be BRAFV600E-positive and later experienced disease stabilization.
Objective responses at the involved sites and patient status at last follow-up
| . | Response to treatment at the involved sites . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # . | Overall response . | Bone . | Cardiovascular system . | Retroperitoneum . | Endocrine system . | CNS . | Lung . | Other . | PET response . | Follow-up duration, (months)* . | Status at last follow-up . | Time on SRL + PDN, (months) . | Treatment at last follow-up . |
| 1 | PR | SD | SD | PR (reduction in peri-renal and mesenteric fibrosis) | — | — | — | Resolution of systemic symptoms and leg edema, no recurrence of bowel obstruction | Not performed | 108 | Alive | 108 | SRL + PDN |
| 2 | PR | SD | Complete resolution of pericarditis; and SD of atrial mass and coated aorta | PR (reduction in peri-renal and peri-aortic fibrosis) | Reduction in serum prolactin (42→17 ng/mL) | — | — | No reappearance of skin nodules | Not performed | 78 | Alive | 78 | SRL + PDN |
| 3 | PR | SD | — | CR (resolution of peri-aortic and mesenteric fibrosis) | — | SD | — | No reappearance of skin nodules | Negative at baseline | 76 | Alive | 76 | SRL + PDN |
| 4 | PR | SD | — | PR (reduction of periaortic and peri-renal fibrosis, and resolution of HDN) | Stable | — | SD | — | Negative at baseline | 70 | Alive | 69 | SRL + PDN |
| 5 | PR | SD | — | PR (reduction of peri-aortic fibrosis and resolution of HDN) | Stable | — | — | — | PMR | 25 | Deceased | 15 | None (withdrew SRL + PDN at month 15 for small-cell lung cancer) |
| 6 | PD | SD | — | — | — | PD | N/A | — | Not performed | 11 | Deceased | 11 | SRL + PDN |
| 7 | PR | SD | — | — | — | PR | — | Stable | Positive at baseline; follow-up PET not performed (lost to follow -up) | 11 | Alive (lost to follow-up) | 11 | SRL + PDN |
| 8 | PD | SD | PD (increase in myocardial infiltration) | PD (worsened HDN and bowel occlusions) | Stable | SD | Stable | Positive at baseline; not repeated for overt clinical progression | 32 | Alive | 32 | Vemurafenib (stopped SRL at month 25 for PD) | |
| 9 | SD | — | — | SD | — | — | — | Stable | SMD | 24 | Alive | 21 | SRL + PDN |
| 10 | SD | SD | SD (with complete resolution of pericardial effusion after drainage) | SD | Stable | — | — | Stable | CMR | 14 | Alive | 14 | SRL + PDN |
| . | Response to treatment at the involved sites . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # . | Overall response . | Bone . | Cardiovascular system . | Retroperitoneum . | Endocrine system . | CNS . | Lung . | Other . | PET response . | Follow-up duration, (months)* . | Status at last follow-up . | Time on SRL + PDN, (months) . | Treatment at last follow-up . |
| 1 | PR | SD | SD | PR (reduction in peri-renal and mesenteric fibrosis) | — | — | — | Resolution of systemic symptoms and leg edema, no recurrence of bowel obstruction | Not performed | 108 | Alive | 108 | SRL + PDN |
| 2 | PR | SD | Complete resolution of pericarditis; and SD of atrial mass and coated aorta | PR (reduction in peri-renal and peri-aortic fibrosis) | Reduction in serum prolactin (42→17 ng/mL) | — | — | No reappearance of skin nodules | Not performed | 78 | Alive | 78 | SRL + PDN |
| 3 | PR | SD | — | CR (resolution of peri-aortic and mesenteric fibrosis) | — | SD | — | No reappearance of skin nodules | Negative at baseline | 76 | Alive | 76 | SRL + PDN |
| 4 | PR | SD | — | PR (reduction of periaortic and peri-renal fibrosis, and resolution of HDN) | Stable | — | SD | — | Negative at baseline | 70 | Alive | 69 | SRL + PDN |
| 5 | PR | SD | — | PR (reduction of peri-aortic fibrosis and resolution of HDN) | Stable | — | — | — | PMR | 25 | Deceased | 15 | None (withdrew SRL + PDN at month 15 for small-cell lung cancer) |
| 6 | PD | SD | — | — | — | PD | N/A | — | Not performed | 11 | Deceased | 11 | SRL + PDN |
| 7 | PR | SD | — | — | — | PR | — | Stable | Positive at baseline; follow-up PET not performed (lost to follow -up) | 11 | Alive (lost to follow-up) | 11 | SRL + PDN |
| 8 | PD | SD | PD (increase in myocardial infiltration) | PD (worsened HDN and bowel occlusions) | Stable | SD | Stable | Positive at baseline; not repeated for overt clinical progression | 32 | Alive | 32 | Vemurafenib (stopped SRL at month 25 for PD) | |
| 9 | SD | — | — | SD | — | — | — | Stable | SMD | 24 | Alive | 21 | SRL + PDN |
| 10 | SD | SD | SD (with complete resolution of pericardial effusion after drainage) | SD | Stable | — | — | Stable | CMR | 14 | Alive | 14 | SRL + PDN |
Objective responses for measurable lesions were reported according to RECIST 1.0 as CR, PR, SD, or PD when assessed on measurable lesions by means of CT or MRI; for bone lesions, considered to be nonmeasurable, we classified responses according to RECIST 1.0 as CR, incomplete response/SD, or PD (see Methods). The response assessed by PET was reported as CMR, PMR, SMD, or progressive metabolic disease (see Methods). For other manifestations (eg, endocrine abnormalities and skin lesions), we provided descriptive responses. PET was performed at baseline and repeated only in patients with pathologic findings.
CMR, complete metabolic response; SMD, stable metabolic disease.
Follow-up duration has been calculated from the start of SRL + PDN treatment to last follow-up.
The remaining 8 patients showed PRs of at least one involved site. Retroperitoneal lesions regressed partially in 4/8 cases and completely in 1/8 (Figure 1 and supplemental Figure 2): at last follow-up, only 2 patients had double-J ureteral stents, and the patient with repeat bowel obstructions (patient #1) no longer developed such complications. Cardiovascular lesions improved in 3/4 cases: the 2 patients with severe pericarditis (one of whom was previously described28 ) underwent pericardiocentesis and remained free of pericardial effusion over the entire follow-up (Figure 1). Bone disease usually remained stable, although an improvement at 99Tc-scintigraphy was observed in 3 cases (Figure 2). CNS lesions progressed in patient #6 and stabilized in patient #3, whereas in patient #7 there was no recurrence of the surgically excised brain mass, and the remaining nodules showed partial regression (supplemental Figure 3). Lung involvement stabilized in all cases. The outcome of the remaining disease manifestations is reported in Table 2. Systemic symptoms improved in most patients; the only patient who experienced persistent systemic manifestations was patient #8. C-reactive protein levels also tended to decrease over time (supplemental Figure 4).
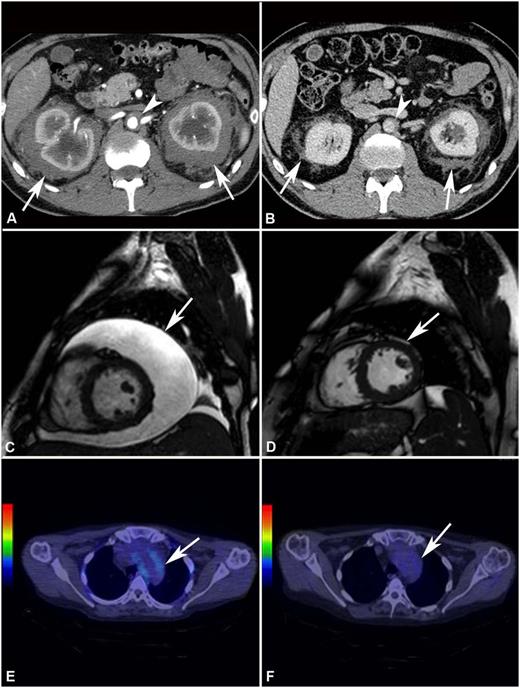
Response to treatment assessed by different imaging modalities. (A) Abdominal CT performed before treatment and (B) after 4 years of SRL and PDN treatment in patient #2. The scans show marked shrinkage of peri-renal (arrows) and peri-aortic fibrosis (arrowhead). (C) Cardiac MRI (T2-weighted, fat-saturation sequence, sagittal view) performed before treatment and (D) after 12 months of treatment with SRL and PDN in patient #10. The scans show neither recurrence of pericardial effusion (arrow) (the patient had also undergone pericardiocentesis) nor signs of pericardial infiltration. (E) 18F-FDG PET-CT performed before and (F) after 12 months of treatment with SRL and PDN in patient #10. The scans (axial view) show disappearance of 18F-FDG uptake at the thoracic aorta level (aortic arch) (arrow).
Response to treatment assessed by different imaging modalities. (A) Abdominal CT performed before treatment and (B) after 4 years of SRL and PDN treatment in patient #2. The scans show marked shrinkage of peri-renal (arrows) and peri-aortic fibrosis (arrowhead). (C) Cardiac MRI (T2-weighted, fat-saturation sequence, sagittal view) performed before treatment and (D) after 12 months of treatment with SRL and PDN in patient #10. The scans show neither recurrence of pericardial effusion (arrow) (the patient had also undergone pericardiocentesis) nor signs of pericardial infiltration. (E) 18F-FDG PET-CT performed before and (F) after 12 months of treatment with SRL and PDN in patient #10. The scans (axial view) show disappearance of 18F-FDG uptake at the thoracic aorta level (aortic arch) (arrow).
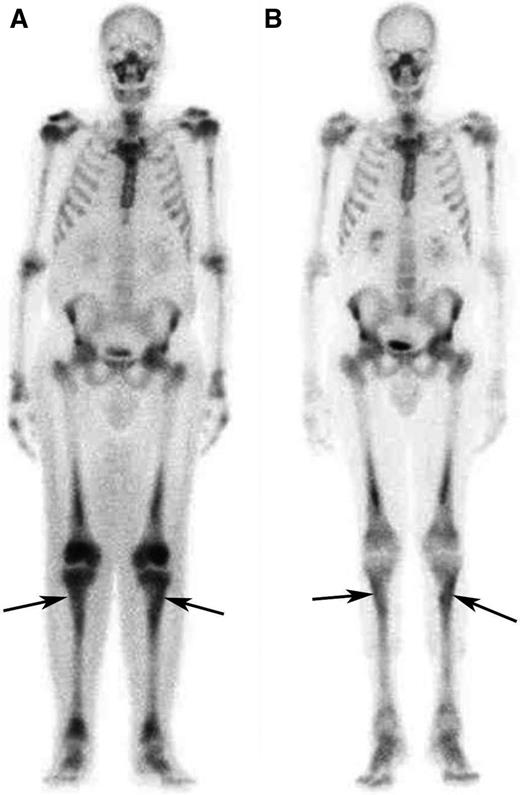
Response to treatment in the long bones assessed by bone scintigraphy. (A) Whole-body 99Tc-bone scintigraphy performed before treatment and (B) after 12 months of treatment with SRL and PDN in patient #5. The scans show a significant reduction in tracer uptake especially in long bones such as the tibias (arrows) and the femurs.
Response to treatment in the long bones assessed by bone scintigraphy. (A) Whole-body 99Tc-bone scintigraphy performed before treatment and (B) after 12 months of treatment with SRL and PDN in patient #5. The scans show a significant reduction in tracer uptake especially in long bones such as the tibias (arrows) and the femurs.
PET-CT was positive at baseline (especially at the bone, vascular, soft-tissue, and retroperitoneal levels) in 5/7 patients studied (Table 2): of these, 1 patient achieved a CMR (Figure 1), 1 a PMR, 1 SD, 1 was lost to follow-up before follow-up PET-CT was performed, and 1 did not repeat PET-CT because of overt clinical progression.
No clinical or laboratory parameters predicted response to therapy. BRAFV600E mutation, detected in 3/6 patients tested, also did not predict particular response patterns.The median follow-up from the start of treatment was 28.5 months (IQR, 16.5-74.5). Two patients died, 1 (patient #6) of progressive CNS disease and the other (patient #5) of metastatic small-cell lung cancer, which developed while ECD was clinically silent; in this patient, SRL and PDN were withdrawn after cancer diagnosis (at month 15), he received chemotherapy (etoposide+cysplatin) and died 10 months later from cancer-related complications (survival analysis of the whole cohort is shown in supplemental Figure 5).
Treatment was generally well tolerated; the main AEs are reported in Table 3. Patient #4 withdrew SRL for 1 month for infectious panniculitis, whereas patient #9 withdrew it for 3 months for interstitial pneumonitis and then resumed it at a lower dose. At last follow-up, all patients were on SRL and PDN except for patient #5 who had developed small-cell lung cancer and patient #8 who had switched to vemurafenib; in all cases, the maintenance PDN dose was 2.5 to 5 mg/day.
AEs occurred during treatment with SRL and PDN
| Patient # . | AEs . |
|---|---|
| 1 | Hypertension (grade 2) |
| 2 | Worsened (preexisting) hyper-triglyceridemia (grade 3) and cushingoid changes (grade 1) |
| 3 | Cushingoid changes (grade 1) |
| 4 | Worsened (preexisting) type 2 diabetes (grade 2), left leg panniculitis (grade 3), and acute coronary syndrome (grade 4) requiring percutaneous angioplasty |
| 5 | Worsened (preexisting) type 2 diabetes (grade 2), bilateral leg edema (grade 2), and cushingoid changes (grade 2) |
| 6 | Cushingoid changes (grade 1) |
| 7 | Hyper-cholesterolemia (grade 1) and hyper-triglyceridemia (grade 2) |
| 8 | Hyper-triglyceridemia (grade 2), cataract (grade 2), and abdominal aorta thrombosis (grade 3) |
| 9 | Interstitial pneumonitis (grade 2) |
| 10 | Hyper-cholesterolemia (grade 1) and hyper-triglyceridemia (grade 2) |
| Patient # . | AEs . |
|---|---|
| 1 | Hypertension (grade 2) |
| 2 | Worsened (preexisting) hyper-triglyceridemia (grade 3) and cushingoid changes (grade 1) |
| 3 | Cushingoid changes (grade 1) |
| 4 | Worsened (preexisting) type 2 diabetes (grade 2), left leg panniculitis (grade 3), and acute coronary syndrome (grade 4) requiring percutaneous angioplasty |
| 5 | Worsened (preexisting) type 2 diabetes (grade 2), bilateral leg edema (grade 2), and cushingoid changes (grade 2) |
| 6 | Cushingoid changes (grade 1) |
| 7 | Hyper-cholesterolemia (grade 1) and hyper-triglyceridemia (grade 2) |
| 8 | Hyper-triglyceridemia (grade 2), cataract (grade 2), and abdominal aorta thrombosis (grade 3) |
| 9 | Interstitial pneumonitis (grade 2) |
| 10 | Hyper-cholesterolemia (grade 1) and hyper-triglyceridemia (grade 2) |
AEs were categorized according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.24
To investigate whether the mTOR pathway is active in ECD lesions, we used IHC, which revealed a diffuse expression of phospho-p70S6K and phospho-mTOR in foamy ECD histiocytes (Figure 3 and supplemental Figure 6). Confocal microscopy showed co-localization of phospho-p70S6K and CD68 (Figure 4), and of phospho-mTOR and CD68 (Figure 4), thus confirming that histiocytes were phospho-p70S6K- and phospho-mTOR–positive. Isotype control staining is shown in supplemental Figure 7.
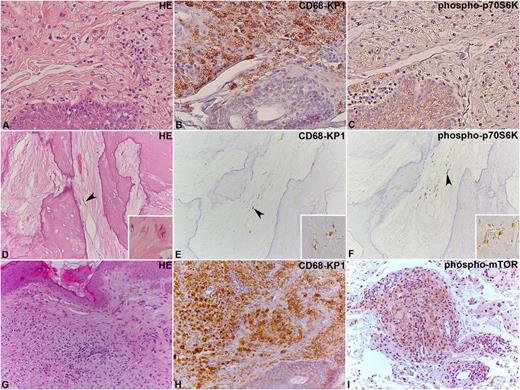
Immunohistochemical analysis of phospho-mTOR and phospho-p70S6K in ECD lesions. (A-C) Two ECD cases located in the skin and (D-F) bone, where the lesional CD68-KP1–positive histiocytes (B,E) show immunoreaction for phospho-p70S6K (C,F). In (D-F), the arrowhead indicates some elongated histiocytes, whose shape results from their encasement by abundant sclerotic fibrous tissue. An additional case of ECD involving the skin (G-I) with CD68-KP1–positive histiocytes (H), which also react with an anti–phospho-mTOR antibody (I) is shown. Original magnification: (A-I), ×20; insets in (D-F), ×40. HE, hematoxylin and eosin.
Immunohistochemical analysis of phospho-mTOR and phospho-p70S6K in ECD lesions. (A-C) Two ECD cases located in the skin and (D-F) bone, where the lesional CD68-KP1–positive histiocytes (B,E) show immunoreaction for phospho-p70S6K (C,F). In (D-F), the arrowhead indicates some elongated histiocytes, whose shape results from their encasement by abundant sclerotic fibrous tissue. An additional case of ECD involving the skin (G-I) with CD68-KP1–positive histiocytes (H), which also react with an anti–phospho-mTOR antibody (I) is shown. Original magnification: (A-I), ×20; insets in (D-F), ×40. HE, hematoxylin and eosin.
Confocal microscopy analysis of phospho-mTOR and phospho-p70S6K in ECD lesions. The same cases as those shown in Figure 3 with immunoreaction for CD68-KP1 (A,D,G), phospho-p70S6K (B,E), phospho-mTOR (H), and relevant merged images (C,F,I). Original magnification: (A-I), ×63.
Confocal microscopy analysis of phospho-mTOR and phospho-p70S6K in ECD lesions. The same cases as those shown in Figure 3 with immunoreaction for CD68-KP1 (A,D,G), phospho-p70S6K (B,E), phospho-mTOR (H), and relevant merged images (C,F,I). Original magnification: (A-I), ×63.
Discussion
ECD often has a progressive, life-threatening course. IFN-α has substantially improved the prognosis of ECD patients, but this treatment is poorly tolerated and often ineffective on cardiac and CNS lesions, although studies using high IFN-α doses showed cardiac and CNS responses.2,27,29 The recent introduction of the BRAFV600E-inhibitor vemurafenib offers an exciting perspective for BRAFV600E-mutated patients; however, the experience with vemurafenib treatment is still limited12,13,30 and no targeted therapy is available for BRAF-wild-type patients. Therefore, alternative treatments are needed for ECD. In this trial, we show that the combination of SRL and PDN generally leads to stabilization or improvement of ECD and induces objective responses in a substantial proportion of patients.
The experience with this therapeutic regimen began with our index case, that we empirically treated with SRL and PDN based on the assumption that ECD has an inflammatory-neoplastic nature. Because he responded brilliantly despite an aggressive clinical presentation, we decided to design this trial. All the enrolled patients had multisystemic involvement and their clinical manifestations were comparable to those reported in other cohorts.2,27 Bone disease, affecting the long bones and in some cases also the facial bones was extremely common (90% of the cases), as well as retroperitoneal involvement (80% of the cases) that caused severe complications such as obstructive uropathy and bowel obstruction. Cardiac and large-vessel disease was detected in 40% of the cases, whereas CNS lesions in only 30%, this manifestation being slightly less frequent than in other series.2,27
Of our 10 patients, 8 had objective responses or disease stabilization, whereas 2 had disease progression. Responses varied at the different sites. We observed significant responses at the retroperitoneal level, with frequent shrinkage of peri-renal and peri-ureteral masses. Patients presenting with severe pericarditis also had an excellent outcome, as they did not show any recurrence of pericardial effusion after pericardiocentesis; however, the course of pericarditis in ECD is still uncertain and it is unknown how often it recurs after pericardiocentesis, thus it is not possible to conclude that the remission of pericarditis was entirely due to our treatment. Responses were heterogeneous at the CNS level, whereas they were marginal or absent at other sites, such as the endocrine system and the bone; however, it must be acknowledged that endocrine abnormalities (eg, diabetes insipidus) in ECD rarely improve,29 and that bone disease does not significantly impact patient prognosis. In parallel with objective response assessment by CT/MRI, we also performed whole-body PET in a fraction of cases; in some patients, we observed partial or CMRs while the corresponding lesions were still unchanged on CT/MRI, which further supports the efficacy of our treatment.
Overall survival is significantly reduced in ECD; per the article by Veyssier-Belot et al, 35 (60%) of the 58 patients whose data were available died, with a mean survival of 19.2 months.21 A series published in 2004 also documented a mortality rate of 60%.31 A more recent analysis of patients largely treated with IFN-α yielded a mortality of 26%.2 Considering these data, our 20% mortality (after a median follow-up of 29 months [IQR, 16.5-74.5] from the start of treatment) appears to be one of the most favorable outcomes reported in the literature. Notably, our therapeutic regimen was also well tolerated, with most patients being able to continue it chronically for several years. This aspect is crucial, since other approaches such as IFN-α, chemotherapy, or even the BRAFV600E-inhibitor vemurafenib can be associated with severe side effects that often preclude their long-term use. Overall, we believe that our treatment regimen might be a feasible option for BRAFV600E-negative patients who have contraindications to IFN-α or who have failed IFN-α; whether our regimen can be considered first-line treatment of BRAFV600E-negative cases cannot yet be determined, and further data are needed.
The mechanisms through which our therapy induces responses are a point of major interest. It is conceivable that responses are largely due to SRL, as it is now known that glucocorticoids are generally ineffective in ECD, except for regimens using high doses that induce improvement of selected manifestations such as exophthalmos.1 The relative inefficacy of glucocorticoids was not clear at the time we designed the trial, and we decided not to change our schedule; additionally, we could not exclude a synergistic effect of SRL and PDN. Future studies based on mTOR-inhibitors given as monotherapy are feasible and could prove useful to address this point.
mTOR is an important regulator of cell growth, proliferation, and apoptosis. In our exploratory analysis, we found signs of activation of the mTOR pathway in ECD lesions: the phosphorylated form of mTOR was intensely expressed on immunohistochemical and immunofluorescence analysis of ECD biopsies, and so was the phosphorylated form of p70S6K, a kinase downstream of mTOR.32 Interestingly, phospho-mTOR and phospho-p70S6K were particularly expressed by CD68+ foamy histiocytes. mTOR signaling proceeds via two different complexes: mTOR-complex 1 (mTORC1) and mTORC2, where mTORC1 is believed to be more rapamycin-sensitive.18 Phospho-p70S6K expression denotes a clear involvement of mTORC1. Multiple factors may contribute to mTOR activation: in a recent study, mutations in NRAS and PIK3CA, which activate mTOR, were detected in 4% and 11% of ECD patients, respectively.14 Probably due to the low number of tested cases, these mutations were negative in our patients. However, other mechanisms involving soluble mediators or cell surface receptors that ultimately switch on the mTOR pathway may be involved and warrant investigation.
Our clinical results are mainly limited by the small size of our patient cohort; however, ECD is extremely rare and our trial is so far the largest prospective investigation in this disease. Additionally, we assessed the major gene mutations identified in ECD in only a fraction of cases, but these findings are very recent and tissue specimens for molecular analysis were often insufficient in our patients.
In conclusion, treatment with SRL and PDN often induces disease stabilization or objective responses in ECD, and is well tolerated; therefore, it represents a valid alternative for patients not candidate to targeted therapies or for those who are intolerant or refractory to IFN-α. The mTOR pathway appears to be active in ECD lesions, particularly in ECD histiocytes.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Prof Rita Gatti for her invaluable help with confocal microscopy analysis, and Drs Cinzia Azzoni and Lorena Bottarelli for their assistance with DNA extraction from the biopsies.
Role of the funding source: The study sponsor had no role in study design, collection, analysis and interpretation of the data, or writing of the manuscript. The corresponding author had full access to all data and took final responsibility for the decision to submit the paper for publication.
Authorship
Contribution: D.G., F.A., C.B., and A.V. designed the study, followed the patients, analyzed the data, and drafted the manuscript; M.N., M.G., D.C., and G. Becchi performed immunohistochemical-immunofluorescence studies and reviewed the diagnostic biopsies; G. Baldari and M.D.F. reviewed the nuclear medicine and radiologic studies, respectively; S.F., G.M., R.F., M.D.G., and G.J. contributed patients to the trial; and R.S. and J.-F.E. performed the molecular analysis of gene mutations. All authors were involved in the critical analysis of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Augusto Vaglio, Nephrology Unit, University Hospital, Via Gramsci 14, 43126 Parma, Italy; e-mail: augusto.vaglio@virgilio.it.
References
Author notes
D.G. and M.N. contributed equally to this study.