Key Points
Maternal sera containing anti-HPA-1a antibodies suppress in vitro megakaryopoiesis through induction of cell death.
The degree of suppression of megakaryopoiesis is variable and is one of the factors determining the severity of neonatal thrombocytopenia.
Abstract
Incompatibility of the human platelet antigen-1 (HPA-1) system is the most common cause of fetal/neonatal alloimmune thrombocytopenia (F/NAIT) and is thought to be mediated by accelerated clearance of antibody-opsonized fetal platelets. We evaluated the effect of maternal sera containing anti-HPA-1a antibodies (F/NAIT sera) on in vitro megakaryopoiesis. Compared with control maternal sera, 14 out of 17 F/NAIT sera significantly reduced megakaryocyte (MK) number. This finding was associated with increased apoptosis and cell death of early MKs/MK progenitors, but normal maturation and differentiation of surviving MKs. An analysis of platelet counts in infants born to mothers following antenatal intravenous immunoglobulin (IVIG) ± prednisone therapy demonstrated a significant and moderately strong correlation between the MK growth in cultures and the infants’ platelet counts at birth. These findings suggest that maternal anti-HPA-1a antibodies can suppress fetal megakaryopoiesis by inducing early cell death and that this influences the neonatal platelet count. Thus, the ability of maternal antibodies to suppress MK growth is a potential predictive factor for the fetal response to maternal IVIG therapy.
Introduction
Incompatibility in the human platelet antigen-1 (HPA-1) system is the most common cause of fetal/neonatal alloimmune thrombocytopenia (F/NAIT).1,2 F/NAIT accounts for the majority of cases of severe thrombocytopenia in full-term neonates. Intravenous immunoglobulin (IVIG) is widely used as postnatal therapy, as well as antenatally in women with a previously affected pregnancy. Current protocols of antenatal IVIG therapy (± steroids) increase the fetal platelet count to >50 × 109/L in the majority of cases, although not usually to normal levels.3
Accelerated clearance of maternal antibody-opsonized fetal platelets is believed to be the major mechanism leading to thrombocytopenia in F/NAIT,4 and it is unclear whether maternal anti-HPA-1a antibodies have any effects on megakaryopoiesis. In adults with autoimmune thrombocytopenia (ITP), suppression of megakaryopoiesis contributes to the thrombocytopenia. This is supported by the finding of apoptotic, para-apoptotic or autophagic features in bone marrow megakaryocytes (MKs),5,6 the in vitro suppression of platelet production by anti-integrin αIIb or β3 autoantibodies from ITP sera,7,8 reduced numbers of reticulated platelets,9 longer than expected survival of antibody-coated platelets,10,11 and the clinical response of ITP patients to thrombopoietic agents.12 We used an in vitro culture system to investigate the effects of sera from pregnant women with anti-HPA-1a antibodies on fetal/neonatal megakaryopoiesis. We also explored the relationship between in vitro suppression of megakaryopoiesis by maternal sera and the platelet counts of the newborn infants, following antenatal treatment with IVIG ± prednisone.
Study design
Serum samples
Samples were obtained from pregnant women with anti-HPA-1a antibodies and prior pregnancies complicated by F/NAIT (n = 17), or with uncomplicated pregnancies (controls, n = 8). F/NAIT samples were collected at 21.2 ± 4.4 weeks of gestation, prior to the initiation of antenatal therapy, in mothers with a previously affected fetus (n = 16), or shortly after delivery of a first affected infant (n = 1).
Cell cultures
CD34+ cells were isolated from type O cord blood collected from healthy full-term neonates. HPA-1a/1a CD34+ cells were cultured with 30 ng/mL thrombopoietin and 10% F/NAIT or control serum.13 MK differentiation, maturation, and ploidy were analyzed by flow cytometry. Details regarding study design, methods, and statistical analysis are provided in the supplemental Methods (available on the Blood Web site).
Results and discussion
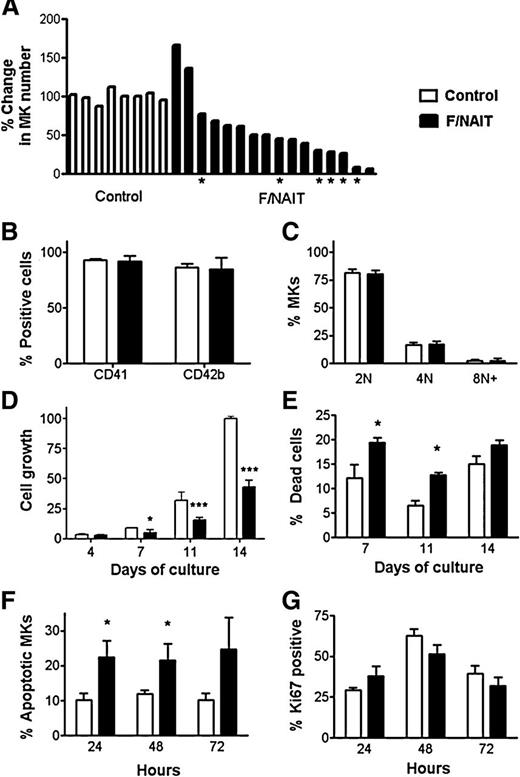
We investigated the effects of F/NAIT maternal sera on in vitro fetal/neonatal megakaryopoiesis, using cord blood–derived HPA-1a/1a CD34+ cells as a source of MKs. To quantify MK proliferation, the number of MKs (CD41+ cells) generated with each sample after 14 days was expressed as a percentage of the mean MK number generated with control sera, set as 100% (see supplemental Methods). Compared with controls, 14 of the 17 F/NAIT sera suppressed in vitro MK generation, with the number of MKs generated ranging from 7% to 77% of controls (Figure 1A). Three of the F/NAIT sera induced increases in megakaryopoiesis: 2 had a moderate stimulatory effect (137% and 166% growth relative to controls; Figure 1A), and 1 was an outlier, generating 10-fold more MKs than control sera (data not shown). This extreme outlier, obtained at 20 weeks gestation from a mother who later delivered twins with platelet counts of 73 and 77 × 109/L, was excluded from further analysis.
Effects of F/NAIT vs control sera on fetal/neonatal megakaryopoiesis. (A-E) Cord blood–derived CD34+ cells were cultured for 14 days in the presence of thrombopoietin and 10% maternal sera (F/NAIT or control). (A) The MK number for each culture was calculated by multiplying cell count and percentage of CD41+ cells. To quantify growth, the number of MKs generated in each culture was expressed as a percentage of the mean MK number in the corresponding control cultures (see details in supplemental Methods). Compared with control sera (n = 8), 14 out of 17 F/NAIT samples caused significant reductions in MK number at the end of culture (day 14). Samples marked with an asterisk were used for the studies shown in Figure 1F-G. (B) CD41 and CD42b surface expression in F/NAIT and control cultures. (C) MK ploidy distribution in F/NAIT and control cultures. (D) Cell numbers were evaluated on days 4, 7, 11, and 14 of culture. The cell number at each different time point was expressed as a percentage of the mean control cell count on day 14 (100%). (E) Percentages of dead cells in the cultures on days 7, 11, and 14, after staining with trypan blue. (F-G) Day 7 cells were treated with 20% of either F/NAIT (n = 6) or control sera (n = 4) for 24, 48, or 72 hours. (F) Percentage of apoptotic MKs after 24, 48, and 72 hours of exposure to F/NAIT or control sera. (G) Percentage of Ki67+ MKs after 24, 48, and 72 hours of exposure to F/NAIT or control sera. Bar graphs indicate the mean ± standard error of the mean of each group. *P < .05; ***P < .001.
Effects of F/NAIT vs control sera on fetal/neonatal megakaryopoiesis. (A-E) Cord blood–derived CD34+ cells were cultured for 14 days in the presence of thrombopoietin and 10% maternal sera (F/NAIT or control). (A) The MK number for each culture was calculated by multiplying cell count and percentage of CD41+ cells. To quantify growth, the number of MKs generated in each culture was expressed as a percentage of the mean MK number in the corresponding control cultures (see details in supplemental Methods). Compared with control sera (n = 8), 14 out of 17 F/NAIT samples caused significant reductions in MK number at the end of culture (day 14). Samples marked with an asterisk were used for the studies shown in Figure 1F-G. (B) CD41 and CD42b surface expression in F/NAIT and control cultures. (C) MK ploidy distribution in F/NAIT and control cultures. (D) Cell numbers were evaluated on days 4, 7, 11, and 14 of culture. The cell number at each different time point was expressed as a percentage of the mean control cell count on day 14 (100%). (E) Percentages of dead cells in the cultures on days 7, 11, and 14, after staining with trypan blue. (F-G) Day 7 cells were treated with 20% of either F/NAIT (n = 6) or control sera (n = 4) for 24, 48, or 72 hours. (F) Percentage of apoptotic MKs after 24, 48, and 72 hours of exposure to F/NAIT or control sera. (G) Percentage of Ki67+ MKs after 24, 48, and 72 hours of exposure to F/NAIT or control sera. Bar graphs indicate the mean ± standard error of the mean of each group. *P < .05; ***P < .001.
The lower MK numbers generated in 14 of 17 F/NAIT cultures were likely the result of early MK progenitor cell death. However, the surviving MKs seemed to develop normally, as suggested by their normal CD41 and CD42b expression levels (Figure 1B) and ploidy distributions (Figure 1C). The variable degrees of suppression of in vitro megakaryopoiesis might have been related to differences in antibody concentration. Antibody titers were available for 8 mothers. Statistical analysis suggested that both the MK growth rate and the platelet count at birth were inversely correlated with antibody titer, but the sample size was too small to reach firm conclusions. Gestation number was also inversely correlated with MK growth, but the correlation was mild and not statistically significant. The variability in our samples was comparable to the study of McMillan et al, in which 12 of 18 ITP plasma samples induced suppression of in vitro MK growth ranging from 26% to 90% of controls.7
Next, we evaluated cell numbers throughout the culture to determine at what stage the differences between F/NAIT and control sera first became apparent in the cultures that induced growth suppression (n = 14). Cell numbers at different days were expressed as percent of the mean cell number in control cultures on day 14 (100%). F/NAIT cultures exhibited significantly lower cell numbers on days 7, 11, and 14, with the mean cell count on day 14 representing 39.9 ± 6.8% of controls (P < .001; Figure 1D). Low cell numbers in F/NAIT cultures were accompanied by significant increases in dead cell percentages on days 7 and 11, by trypan blue staining (P < .05; Figure 1E). Consistently, treatment of day 7 cells (43 ± 5% CD41+) with 20% F/NAIT sera (n = 6) for 24 or 48 hours nearly doubled the percentage of apoptotic MKs compared with control sera (n = 4, P < .05; Figure 1F). MK proliferation, measured by Ki67 staining, was similar in F/NAIT and control cultures (Figure 1G). These findings suggested that the suppressive effects of F/NAIT sera on megakaryopoiesis were the result of induced cell death, rather than inhibition of proliferation. Consistently, Yougbaré et al recently reported that human anti-HPA-1a antibodies increased proapoptotic signaling on endothelial cells.14 Antibodies against glycoprotein IIb/IIIa have also been shown to induce MK death in ITP, and in the setting of eptifibatide-induced thrombocytopenia.5,15
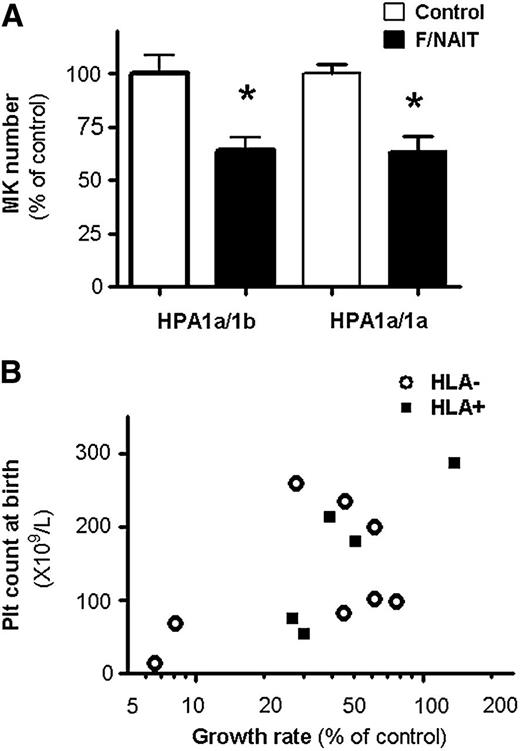
Because neonates with F/NAIT are born to parents with HPA-1 incompatibility, their genotype is invariably HPA-1a/1b. Thus, we explored the effects of F/NAIT sera on HPA-1a/1b MK progenitors. Compared with control sera (n = 3), F/NAIT sera (n = 4) reduced the cell number in HPA-1a/1b cultures by 35.8 ± 12.1%, an effect similar to that observed on HPA-1a/1a cells (Figure 2A). There were again no differences in CD41 and CD42b expression or ploidy between F/NAIT and control cultures (data not shown).
Effects of F/NAIT sera on HPA-1a/1b MKs and correlation between MK growth rates and platelet counts at birth. (A) Exposure to F/NAIT sera for 7 days caused similar decreases in the number of MKs generated from HPA-1a/1b and HPA-1a/1a cultures, compared with control sera. *P < .05. (B) Platelet counts at birth in neonates vs growth rate of MKs cultured in maternal sera (relative to controls). Samples known to have antibodies against class I HLA antigens are indicated by filled squares.
Effects of F/NAIT sera on HPA-1a/1b MKs and correlation between MK growth rates and platelet counts at birth. (A) Exposure to F/NAIT sera for 7 days caused similar decreases in the number of MKs generated from HPA-1a/1b and HPA-1a/1a cultures, compared with control sera. *P < .05. (B) Platelet counts at birth in neonates vs growth rate of MKs cultured in maternal sera (relative to controls). Samples known to have antibodies against class I HLA antigens are indicated by filled squares.
Finally, we analyzed the correlation between the degree of suppression of megakaryopoiesis by maternal serum and neonatal platelet count at birth, following antenatal IVIG ± prednisone therapy. Samples obtained prior to the initiation of antenatal therapy in mothers with history of a previously affected fetus, and in whom the platelet count of the current infant at birth was known, were included in this analysis (n = 13; 11 IVIG + prednisone, 2 IVIG alone). As shown in Figure 2B, we found a significant correlation between in vitro MK growth values and infants’ platelet counts at birth, suggesting that suppression of megakaryopoiesis is one of the factors contributing to the thrombocytopenia (Spearman correlation 0.57, P = .04; Pearson correlation 0.59, P = .03, using log transformation to normalize distribution of growth rates). Antibodies against class I HLA antigens were present in 5 samples but did not influence MK growth (P > .40; Figure 2B). These findings were consistent with a single prior study, in which the degree of inhibition induced by sera from 3 mothers with anti-HPA-1a antibodies on MK colony formation correlated with the severity of the fetal thrombocytopenia.16
In conclusion, we found that most F/NAIT maternal sera suppress in vitro megakaryopoiesis, primarily by inducing cell death. Furthermore, the degree of MK growth in culture correlated with the infant’s platelet count at birth, following antenatal therapy with IVIG ± prednisone. These findings suggest that the ability of maternal antibodies to suppress MK growth is a potential predictive factor for the fetal response to maternal IVIG therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Brian R. Curtis from the Blood Center of Wisconsin for his help retrieving data from archived antibody titer records, and the pregnant mothers that generously contributed their samples to this study.
This work was supported by grants from the National Institutes of Health, Heart, Lung and Blood Institute (RO HL069990 [M.S.-V.] and PO1HL046925) and a William Randolph Hearst Foundation Award for Peri- and Prenatal Medicine (Z.-J.L.).
Authorship
Contribution: Z.-J.L. designed and performed experiments, collected and analyzed data, and wrote the manuscript; J.B.B., M.L., J.G.M., and C.G. collected and processed serum samples from F/NAIT mothers, provided clinical data, and assisted with the preparation of the manuscript; F.F.-M. and C.C. obtained serum samples from healthy control mothers, collected cord blood samples, and performed experiments; H.A.F. contributed statistical analysis and interpretation; and M.S.-V. supervised and designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martha Sola-Visner, Children’s Hospital Boston, Division of Newborn Medicine, 300 Longwood Ave, Enders Research Building, Room 961, Boston, MA 02115; e-mail: martha.sola-visner@childrens.harvard.edu; and Zhi-Jian Liu, Children’s Hospital Boston, Division of Newborn Medicine, 300 Longwood Ave, Enders Research Building, Room 950, Boston, MA 02115; e-mail: zhi-jian.liu@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal