To the editor:
Transcranial Doppler (TCD) screening and intensification therapy may reduce the risk of stroke from 11% to 1.9% in children with sickle cell anemia (SCA).1 However, the relatively low specificity of TCD screening in identifying individuals at risk of stroke2 highlights the need for prognostic tools with enhanced accuracy to enable the identification of individuals at the highest risk. Recently, Flanagan et al3 reported the association of GOLGB1 Y1212C (rs3732410) and ENPP1 K173Q (rs1044498) mutations with a decreased risk of stroke, whereas PON1 Q192R (rs662) was associated with an increased risk. Thus, the present study was undertaken to evaluate the influence of these mutations on the risk of developing overt ischemic stroke (hereafter referred to as “stroke”) in a newborn cohort from Minas Gerais, Brazil.
Approval was obtained from the Hemominas Foundation institutional review board. Informed consent was provided according to the Declaration of Helsinki. The study included 395 hemoglobin SS-genotyped children aged 6 to 16 years who were followed-up at the Hemominas Foundation from birth until December 31, 2014. Stroke was defined as an acute neurological deficit lasting >24 hours. Diagnosis was confirmed in all children by imaging assessments. Our study also analyzed high-risk TCD as a second outcome, and this was defined as a time-averaged mean of the maximum velocity (TAMMX) ≥200 cm/s in the internal carotid or middle cerebral artery. Children who had suffered stroke as well as those for whom TCD screening was inadequate were excluded from the second analysis. Polymorphisms were detected by polymerase chain reaction/restriction fragment length polymorphism and confirmed by DNA sequencing in at least 5% of random samples.
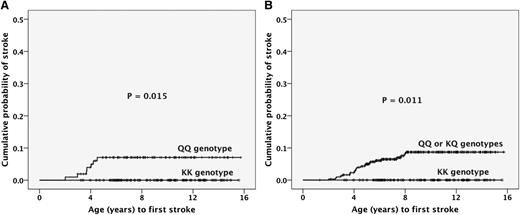
Of the 395 children, 23 (5.8%) had stroke. The frequency of stroke was null (0 of 83) for children who harbored the ENPP1 KK genotype, 6.8% (7 of 103) for those with QQ genotype, and 7.7% (16 of 209) for children with KQ genotype (P = .037). The cumulative probability of stroke was significantly (P = .015) higher for children with the QQ genotype than for those with the KK genotype (Figure 1A). Similar results were obtained comparing QQ/KQ genotypes as 1 group with the KK group (Figure 1B). Of the 333 children with valid TCD, 34 (10.2%) had high-risk readings. The frequency of high-risk TCD was 5.2% (4 of 77) for the KK genotype, 11.2% (19 of 169) for the KQ genotype, and 12.6% (11 of 87) for the QQ genotype. The cumulative probability of high-risk TCD was higher for children with the QQ genotype than for those with the KK genotype, but not significantly (P =.09). The TAMMX of children with the QQ genotype was significantly higher than that of those with the KK genotype (P =.018). No association between GOLGB1 Y1212C or PON1 Q192R and stroke or high-risk TCD was detected (data not shown).
Cumulative probability of stroke according to ENPP1 K173Q genotype. (A) The cumulative probability for the KK genotype group was 0% whereas that for the QQ group was 7.1% (SE, 2.6%; P = .015). (B) The cumulative probability for the KK genotype group was 0% whereas that for the QQ or KQ group was 8.7% (SE, 1.8%; P = .011). Function: (1-survival), according to the Kaplan-Meier method. SE, standard error.
Cumulative probability of stroke according to ENPP1 K173Q genotype. (A) The cumulative probability for the KK genotype group was 0% whereas that for the QQ group was 7.1% (SE, 2.6%; P = .015). (B) The cumulative probability for the KK genotype group was 0% whereas that for the QQ or KQ group was 8.7% (SE, 1.8%; P = .011). Function: (1-survival), according to the Kaplan-Meier method. SE, standard error.
In contrast with the results of Flanagan et al3 for whom ENPP1 Q173 was associated with a decreased risk of stroke, our findings showed instead that ENPP1 K173 was the “protective” variant. Our data further showed that only children with the KK genotype are “protected,” suggesting the dominance of the Q allele. The K173Q variant (frequently reported as K121Q because of an incorrect assignment at the start codon in early studies on ENPP1) is located in the second somatomedin-B-like domain, a noncatalytic ectodomain of ENPP1 that mediates protein homodimerization. The Q173 variant has been associated with insulin resistance,4 which is a condition that predisposes to cardiovascular events5 and type 2 diabetes.6 In addition, an analysis showed that the Q173 variant was significantly associated with increased blood pressure,7 which is a known independent risk factor for the development of stroke in individuals with SCA.8 Furthermore, the Q173 variant has been significantly associated with reduced nitric oxide (NO) synthase activity in human endothelial cells.7 NO lower bioavailability may be considered a possible pathogenic mechanism by which the ENPP1 K173Q modulates the risk of stroke in children with SCA. Evidence supporting this hypothesis includes that KK children had a significantly lower hemolytic rate, as measured by lower reticulocyte count and higher total hemoglobin concentration (data not shown) than QQ children. This would lead to reduced destruction rate of L-arginine, the substrate for NO production, as well as reduced scavenging of NO by free hemoglobin.
In this study, we did not detect any protective association between the GOLGB1 Y1212C mutation and the risk of stroke, as proposed by Flanagan et al.3 In this respect, although it is considered that a cohort-designed study may be more reliable than other types of study for detecting genuine associations, it is possible that we failed to notice the modest effect exerted by GOLGB1 Y1212C on the risk of stroke.
Consistent with the data of other investigators,9 our study found no association between the PON1 Q192R mutation and stroke. Analyses using the PROVEAN10 and SIFT11 algorithms suggest that there would be no impairment of protein function, which supports our results. Furthermore, in a meta-analysis study, PON1 Q192R was found to have no effect on the risk of ischemic stroke in 6 populations of individuals without SCA.12
In conclusion, using longitudinal analysis, we have shown that the Q genetic variant of the ENPP1 K173Q mutation in children with SCA was associated with an increased risk of stroke and, possibly, with the development of high-risk TCD readings.
Authorship
Acknowledgments: The authors acknowledge all subjects and parents for their cooperation in the study.
This work was supported by Fundação Hemominas, Núcleo de Ações e Pesquisa em Apoio Diagnóstico da Universidade Federal de Minas Gerais, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (PPM-00266-13), Conselho Nacional de Desenvolvimento Científico e Tecnológico (304530/2011-5), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Contribution: A.R.B. designed the study, performed genotyping tests, collected and analyzed data, and wrote the manuscript; R.R.S. and N.E.T. performed genotyping tests and commented on the manuscript; C.V.-R. performed bioinformatic analysis and commented on the manuscript; C.M.S. performed TCD measurements, collected data, and commented on the manuscript; and M.B.V. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: André Rolim Belisário, Centro de Tecidos Biológicos de Minas Gerais, Fundação Hemominas, Rua das Goiabeiras, 779, Lagoa Santa, Minas Gerais, Brazil 33400-000; e-mail: andrebelisario@yahoo.com.br.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal