In this issue of Blood, 2 independent reports by Rapetti-Mauss et al and Glogowska et al describe the first example of a human disease: a novel type of hemolytic hereditary xerocytosis (HX) that is caused by a genetic defect in the Gardos channel, a Ca2+-sensitive K+ channel critical for red cell volume regulation, survival, and function.1,2
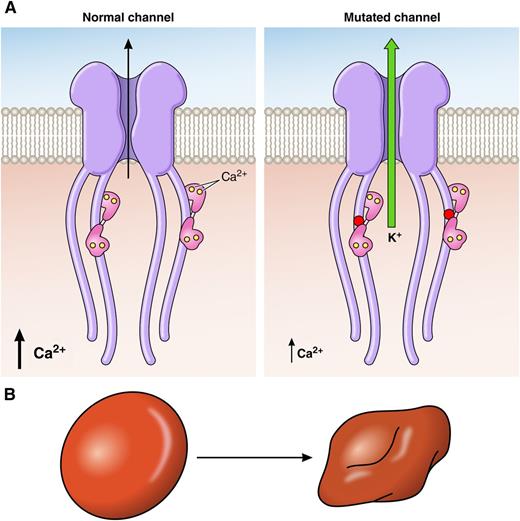
Mutated Gardos channel is activated by 10 times lower Ca2+ concentration and shows abnormally slow inactivation kinetics. These combined effects lead to excessive loss of K+ (A) and red blood cell dehydration (B), which is associated with hemolytic anemia. The disease causing the Arg352His mutation (red dot) is located in the highly conserved region of the calmodulin binding domain of the Gardos channel; calmodulin with bound Ca2+ ions is shown in pink. The other disease-causing mutations identified as Val282Met and Val282Glu are located in the predicted transmembrane loop S6 of the channel. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mutated Gardos channel is activated by 10 times lower Ca2+ concentration and shows abnormally slow inactivation kinetics. These combined effects lead to excessive loss of K+ (A) and red blood cell dehydration (B), which is associated with hemolytic anemia. The disease causing the Arg352His mutation (red dot) is located in the highly conserved region of the calmodulin binding domain of the Gardos channel; calmodulin with bound Ca2+ ions is shown in pink. The other disease-causing mutations identified as Val282Met and Val282Glu are located in the predicted transmembrane loop S6 of the channel. Professional illustration by Patrick Lane, ScEYEnce Studios.
Almost 60 years ago, the Hungarian physiologist Gyorgy Gárdos described a Ca2+-dependent K+ loss from human red cells.3 Since then, the Gardos channel that is responsible for this phenomenon has been an object of curiosity and intense investigations, serving as a model for a broader physiologic perspective and an intriguing component of red cell function.4 It has been receiving increasing attention in recent years as an important player in red cell volume homeostasis. Cell dehydration is established as a primary disorder associated with inherited anemias such as HX, but more commonly, it is a secondary effect that accompanies various other red cell disorders, eg, sickle cell disease and β-thalassemia. The molecular and genetic bases of red cell primary hydration disorders are poorly understood. Mutations in the mechanosensitive nonselective cation channel PIEZO1 have been recently identified as a cause of HX. The present reports demonstrate for the first time that some forms of HX could stem from mutation in the Gardos channel, constituting the first example of red cell channelopathy involving this protein.
Red cell hydration status is determined by complex interactions between solutes, membrane ion channels, pumps, and transporters. Red cell volume is primarily controlled via regulation of monovalent cation content, and its net reduction leads to cell dehydration and concomitant increased mean corpuscular hemoglobin concentration and decreased osmotic fragility.5 The exact mechanisms by which recently described mutations in PIEZO1 result in red blood cell (RBC) dehydration are yet to be defined.6 Gain-of-function mutations may have direct effect on PIEZO1-mediated Na+/K+ fluxes and alteration of red cell cation content. Alternatively, indirect mechanism may involve PIEZO1-mediated Ca2+ influx and downstream activation of the Ca2+-activated K+ efflux via the Gardos channel, leading to subsequent dehydration of red cells.7 The 2 present reports add to the list of known genetic causes of HX by demonstrating that some forms of HX could arise directly from mutations in the Gardos channel itself, thus strengthening its key role in RBC dehydration pathologies.
Rapetti-Mauss et al identified, in 2 unrelated HX affected families, a dominantly inherited mutation in the KCNN4 gene encoding the Gardos channel. The missense mutation was located to a highly conserved region interacting with calmodulin on the C terminus of the channel protein. Glogowska et al identified, in 2 other unrelated families, a different mutation in the predicted transmembrane loop S6 of the Gardos channel. Importantly, Rapetti-Mauss et al, in functional studies with a mutated channel expressed in Xenopus oocytes and mammalian HEK cells, showed that it is activated by a 10 times lower Ca2+ concentration (see figure panel A) and shows abnormally slow inactivation kinetics compared with the normal channel. These combined effects lead to excessive loss of K+ and dehydration of diseased red cells (see figure panel B) that is associated with hemolytic anemia. This finding is reminiscent of the pathogenic mechanism associated with several mutations affecting PIEZO1, where slowing inactivation kinetics of this channel produces more Ca2+ influx and excessive loss of K+.
Although the identification of mutations in KCNN4 leading to red cell dehydration in HX by 2 independent groups is a cause for satisfaction and opens new avenues of research toward a further understanding of red cell volume regulation, some questions remain unanswered. What is the mechanistic basis for what appears to be significant differences in the clinical phenotype due to mutations in the different domains of the channel? Are there different extents of cell dehydration due to these different mutations?
These studies provide a substantial step forward toward developing a comprehensive understanding of the mechanistic basis for red cell volume homeostasis in health and disease. These findings also raise the possibility that HX due to Gardos channel mutations could be treated by with specific Gardos channel inhibitors. Senicapoc is one such promising inhibitor that is well tolerated and showed improvement of sickle cell survival in a clinical setting.8 These findings will also be useful to establish an HX genotype/phenotype relationship and to refine the classification of RBC cation leak disorders on a molecular basis as discussed in earlier studies in the context of PIEZO1 mutations.9
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal