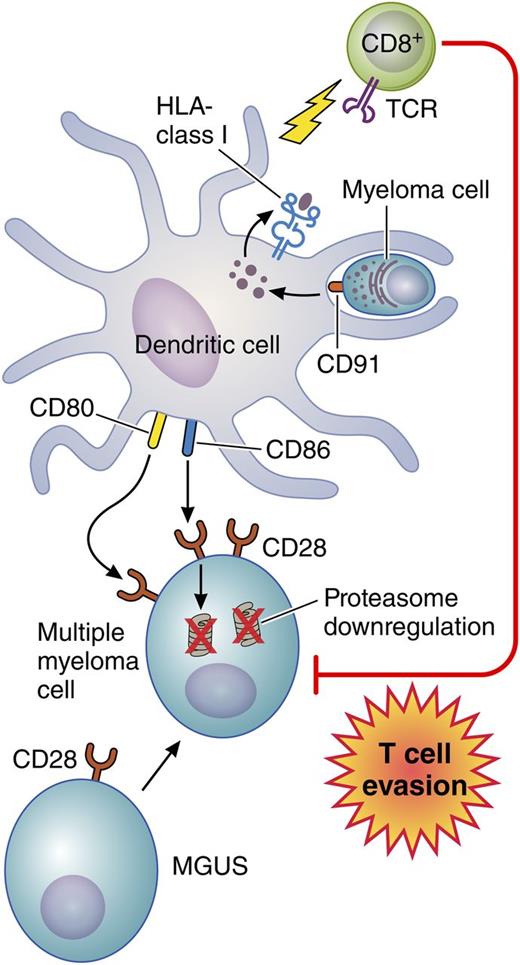
In this issue of Blood, Leone et al describe a novel mechanism mediated by bone marrow dendritic cells (DCs) that impairs T-cell recognition and killing of myeloma cells.1
Bone marrow DCs engulf apoptotic myeloma cells via CD91. Myeloma tumor antigens are then processed and presented on class I HLA molecules to activate infiltrating CD8+ T cells. At the same time, using CD80/CD86, these DCs interact with nonapoptotic myeloma cells that express higher levels of CD28 compared with plasma cells from patients with monoclonal gammopathy of undetermined significance (MGUS). As a result, proteasome degradation occurs, which impairs tumor antigen expression on the myeloma cells thereby rendering them more resistant to T-cell recognition and killing. Professional illustration by Patrick Lane, ScEYEnce Studios.
Bone marrow DCs engulf apoptotic myeloma cells via CD91. Myeloma tumor antigens are then processed and presented on class I HLA molecules to activate infiltrating CD8+ T cells. At the same time, using CD80/CD86, these DCs interact with nonapoptotic myeloma cells that express higher levels of CD28 compared with plasma cells from patients with monoclonal gammopathy of undetermined significance (MGUS). As a result, proteasome degradation occurs, which impairs tumor antigen expression on the myeloma cells thereby rendering them more resistant to T-cell recognition and killing. Professional illustration by Patrick Lane, ScEYEnce Studios.
A small but growing population of patients who remain in complete remission and are progression free at 10 years after diagnosis of multiple myeloma (MM) is emerging as a result of the use of the “total therapy” concept pioneered by Barlogie et al.2 Total therapy uses highly active myeloma first-line therapies followed by 1 or 2 autologous stem cell transplantations (SCTs) and consolidation/maintenance therapy. Still only about 30% of MM patients are cured according to these criteria, and a much smaller fraction of patients with high-risk disease reach these milestones. Initial enthusiasm for an immunotherapeutic approach to MM based on evidence for a graft-versus-myeloma effect in the setting of allogeneic SCT has been tempered by the high risks of morbidity and mortality from graft-versus-host disease and by the higher-than-expected rate of relapse.3,4 Mechanisms of immune evasion by MM cells are variable but are likely to include reduced expression of HLA molecules, reduced expression of tumor antigen peptides, enhanced expression of inhibitory ligands such as programmed cell death ligand 1 (PD-L1) and PD-L2, and recruitment of counterregulatory cells such as T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs).
More recent work on immunotherapy for MM has centered on strategies for generating an autologous graft-versus-myeloma effect and is based on observations that myeloma-reactive T cells with cytotoxic potential after activation are present at low frequencies in the marrow and blood of MM patients.5 Promising approaches include administration of MM-DC fusion vaccines, adoptive transfer of activated marrow-infiltrating lymphocytes, and infusions of genetically modified autologous T cells engineered to express an affinity-enhanced T-cell receptor (TCR) for myeloma tumor antigens, including the cancer-testis antigens NY-ESO-1 and LAGE-1.6-8 These approaches have generally used autologous SCT as a platform because the lymphopenia and lower tumor burden following high-dose chemotherapy may promote homeostatic proliferation of myeloma-reactive lymphocytes while reducing the burden of immunosuppressive cells such as Tregs and MDSCs.
The bone marrow microenvironment plays a critical role in support of myeloma cell growth and resistance to apoptosis and homing as a result of the elaboration of cytokines such as interleukin-6 (IL-6), C-X-C motif chemokine 12 (CXCL12), insulinlike growth factor 1 (IGF-1), vascular endothelial growth factor A (VEGF-A), and many others by mesenchymal stem cells, osteoblasts, osteoclasts, vascular endothelial cells, adipocytes, and fibroblasts.9 In addition, the marrow represents a complex immunologic milieu that both attracts and modulates the function of tumor-reactive immune cells.
The success of many of these novel immunotherapeutic approaches for myeloma depends on effective trafficking of myeloma-specific T cells to the marrow and on their persistence and functionality. Leone et al1 provide insight into a novel mechanism whereby the marrow microenvironment may promote immune evasion (see figure). By using marrow samples from 20 patients with MGUS and 20 patients with symptomatic MM, they found that both myeloid and plasmacytoid DCs accumulate in the marrow during the transition from MGUS to MM. Furthermore, these DCs engulf apoptotic myeloma cells through recognition of calreticulin (CD91), an “eat me” signal on the myeloma cells, and then, through tumor antigen processing, they activate myeloma-specific marrow-infiltrating CD8+ T cells. The latter was demonstrated by analyzing the subset of samples from 4 patients carrying HLA-A*0201 for the level of CD8+ T cells specific for the HLA-A*0201-restricted NY-ESO-1157-165 epitope. The authors showed that the level of NY-ESO-1157-165-specific CD8+ T cells found in the marrow of all 4 of these patients became significantly lower after CD91 blockade, which prevented the DCs from engulfing the myeloma cells and presenting tumor antigen peptides to the CD8+ T cells. Conversely, by using surface CD80/CD86, these same DCs interacted with nonapoptotic myeloma cells through CD28, a major T-cell costimulatory molecule that is expressed at higher levels in plasma cells from MM patients than from MGUS patients and that mediated a significant downregulation of expression of proteasome subunits. Because the proteasomes are critical for tumor antigen processing, this would be expected to decrease expression of tumor antigen peptides on the myeloma cells, enabling them to evade CD8+ T-cell recognition and killing. Indeed, by using flow-based cytotoxicity assays, the CD8+ T cells that expanded in the presence of autologous DCs preloaded with apoptotic myeloma cells successfully killed myeloma cells that were preincubated with DCs across a Transwell barrier, not exposed to DCs, or exposed to DCs in the presence of a CD28-blocking antibody. However, these primed and expanded CD8+ T cells did not kill the myeloma targets if the myeloma cells were preincubated directly with bone marrow DCs. Importantly, this mechanism adds to earlier observations suggesting that CD28 expression on MM cells also induces chemotherapeutic resistance.10
Important questions remain, including whether this finding of CD28-CD80/CD86-mediated immune evasion can be translated to the clinic by using CD28 blocking agents such as CTLA4-immunoglobulin or inhibitors of CD28 downstream signaling through the PI3K-AKT pathway to restore immune sensitivity. Conceivably, T cells engineered to express myeloma-directed chimeric antigen receptors (CARs) may be able to bypass this specific immuno-inhibitory pathway if the expression of the specific CAR target is not affected by proteasome loss. T cells engineered to express large numbers of affinity-enhanced TCRs may also compensate for reduced expression of tumor antigen peptides. Finally, checkpoint inhibitors such as programmed cell death 1 (PD-1), PD-L1, or CTLA-4 antibodies may partially offset immunosuppressive signals from the marrow microenvironment. Nonetheless, the study by Leone et al1 highlights the necessity to address defects in both the effectors and the myeloma targets to optimize the efficacy of immunotherapy for MM. It is likely that the most effective immunotherapeutic approach for MM will include strategies for expanding the repertoire, function, and persistence of myeloma-directed T cells as well as enhancing the sensitivity of MM cells to immune attack.
Conflict-of-interest disclosure: The author declares no competing financial interests.