Key Points
MKL1 deficiency results in actin cytoskeletal disruption in myeloid and lymphoid cell lineages.
MKL1 deficiency impairs neutrophil migration associated with downregulation of myosin II.
Abstract
Megakaryoblastic leukemia 1 (MKL1), also known as MAL or myocardin-related transcription factor A (MRTF-A), is a coactivator of serum response factor, which regulates transcription of actin and actin cytoskeleton–related genes. MKL1 is known to be important for megakaryocyte differentiation and function in mice, but its role in immune cells is unexplored. Here we report a patient with a homozygous nonsense mutation in the MKL1 gene resulting in immunodeficiency characterized predominantly by susceptibility to severe bacterial infection. We show that loss of MKL1 protein expression causes a dramatic loss of filamentous actin (F-actin) content in lymphoid and myeloid lineage immune cells and widespread cytoskeletal dysfunction. MKL1-deficient neutrophils displayed reduced phagocytosis and almost complete abrogation of migration in vitro. Similarly, primary dendritic cells were unable to spread normally or to form podosomes. Silencing of MKL1 in myeloid cell lines revealed that F-actin assembly was abrogated through reduction of globular actin (G-actin) levels and disturbed expression of multiple actin-regulating genes. Impaired migration of these cells was associated with failure of uropod retraction likely due to altered contractility and adhesion, evidenced by reduced expression of the myosin light chain 9 (MYL9) component of myosin II complex and overexpression of CD11b integrin. Together, our results show that MKL1 is a nonredundant regulator of cytoskeleton-associated functions in immune cells and fibroblasts and that its depletion underlies a novel human primary immunodeficiency.
Introduction
Primary immunodeficiency (PID) resulting from disorders of neutrophil function predispose to bacterial and fungal infections. Defined mechanisms include defects of respiratory burst, integrin activation, and neutrophil granules,1 but many conditions in this heterogeneous group of PIDs remain uncharacterized. Although actin regulation is known to be central to several neutrophil functions, such as phagocytosis, adhesion, and migration, cytoskeletal defects causing neutrophil disorders in humans are rare. Mutations in the cytoskeletal regulators Rac2 and Wiskott-Aldrich syndrome protein (WASp) result in immunodeficiencies associated with reduced filamentous actin (F-actin) assembly from globular actin (G-actin), specifically in hematopoietic cells. Although both proteins are expressed in neutrophils, only Rac2 deficiency produces clinically significant neutrophil dysfunction,2 highlighting the importance of specific cytoskeletal regulators in this cell type.
Megakaryoblastic leukemia 1 (MKL1) and MKL2 are members of the myocardin-related transcription factor (MRTF) protein family.3,4 MRTFs are held in an inactive state in the cytoplasm in a reversible complex with G-actin. Stimulation of Rho GTPases promotes incorporation of G-actin into F-actin filaments, releasing MRTFs from G-actin and allowing their import to the nucleus, where MRTFs function as coactivators of serum response factor (SRF) and stimulate SRF-mediated transcription of actin and actin cytoskeleton–related genes.5,6 In contrast with myocardin, which is restricted to cardiomyocytes and smooth muscles cells, MKL1 and MKL2 are widely expressed.3 Although MKL2- and myocardin-knockout mice are embryonically lethal, MKL1-knockout mice have a less severe phenotype that demonstrates premature involution of mammary glands, partial embryonic lethality due to abnormal cardiogenesis associated with myocardial cell death, and reduced platelet counts in peripheral blood.7,8 Several studies indicate that MKL1 is required for maturation and migration of megakaryocytes,8-10 and its absence can only partially be compensated for by the presence of MKL2. However, the role of MKL1 in myeloid or lymphoid lineage immune cells has not been reported. Here we describe, for the first time, human MKL1 deficiency caused by a homozygous mutation in a child with severe bacterial infections. Loss of MKL1 resulted in low levels of F-actin and impaired cytoskeletal functions of neutrophils and myeloid lineage dendritic cells (DCs) as well as lymphoid lineage cells. These findings define MKL1 deficiency as a novel PID and elucidate a nonredundant role for MKL1 in human immune cells.
Material and methods
Human neutrophil isolation and peripheral blood DC cultures
Blood samples were obtained with informed consent from the parents of the patient in accordance with the Declaration of Helsinki and with approval from local ethics committees (04/Q0501/119 and 06/Q0508/16). Neutrophils were isolated from healthy donor or patient blood. Briefly, 2 mL of a 5% dextran-saline solution was added to 10 mL of blood and gently mixed by inversion before being left to sediment for 30 minutes. The plasma layer was collected and overlaid on an equal volume of Ficoll-Paque reagent and then centrifuged at 1800 rpm for 10 minutes. The peripheral blood mononuclear cell (PBMC) layer was taken for CD14+ cell selection, and the neutrophil pellet was resuspended in distilled water for 20 seconds before addition of 2× saline solution to restore isotonicity. The neutrophils were then centrifuged at 1200 rpm for 7 minutes and resuspended in warm complete RPMI.
CD14+ cells were magnetically labeled and positively selected on LS columns (Miltenyi Biotec). The column flowthrough, representing the unlabeled lymphocyte fraction, was collected for further experiments. CD14+ cells were eluted from the column and seeded on day 0 at 1 × 106 cells per well in 6-well plates in complete growth medium (RPMI + 10% fetal calf serum [FCS] + Pen/Strep) supplemented with 100 ng/mL recombinant human granulocyte macrophage colony-stimulating factor and 25 ng/mL recombinant human interleukin-4. Fresh cytokines were added on day 3, and cells were harvested for use on day 6 or 7.
THP1 and HL-60 cell culture
THP1 cells were cultured in suspension in RPMI-Glutamax supplemented with 10% FCS and Pen/Strep. Cells were maintained at 0.5 to 1 × 106 cells/mL. For DC differentiation, THP1 cells were cultured in the presence of 10 ng/mL recombinant human interleukin-4 and 10 ng/mL recombinant human granulocyte macrophage colony-stimulating factor for 6 days. Cells were split on days 2 and 5 with addition of fresh cytokines. Both adherent and suspension cells were harvested for use on day 6 or 7.
HL-60 cells were maintained in culture at a concentration between 0.1 and 1.5 × 106 cells/mL in RPMI supplemented with 10% FCS and 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid. To obtain differentiated neutrophil-like cells (dHL-60), 1 × 106 HL-60 cells were cultured for 5 days in media supplemented with 1.3% dimethylsulfoxide in a T25 flask.
Migration experiments
Depending on the cells used for the experiment, coverslips were coated with a solution of fibrinogen at 25 mg/mL (primary neutrophils) or with a solution of fibronectin at 100 μg/mL (dHL-60) for 1 hour at 37°C. The coverslips were washed twice with phosphate-buffered saline before being used, and dHL-60 cells were washed 3 times in a warm Hanks balanced salt solution/2% human albumin/100 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid solution. Next, 1 × 105 neutrophils or dHL-60 cells were left to adhere to the coated coverslip for 20 to 30 minutes. The coverslip was then washed once with the media used for the migration without chemokine to remove nonadherent cells. A 100-nM formyl methionyl leucyl phenylalanine (fMLP) solution was used for experiments with neutrophils/dHL-60 cells. To stabilize the chemokine gradient in the Dunn chamber, 80 μL of 1% agarose gel containing the chemokine was poured into the outer well of the chamber and left to polymerize for 2 minutes. The inner well was filled with 50 μL of the media without chemokine, and then the chamber was assembled by laying down the coverslip on which the cells were adhering. The chamber was then sealed with wax. Neutrophils/dHL-60 cells were then imaged at 37°C on an Axiovert 135 microscope equipped with a motorized stage that captured 1 image per minute for 1 hour.
Migration of Epstein-Barr virus–transformed lymphoblastoid cell lines was performed in µ-Slide Chemotaxis3D (Ibidi) collagen matrix according to the manufacturer’s instructions. Cells were stimulated with MIP-3α (100 ng/mL) 1 hour prior to imaging. Images were taken over 2 hours on a Leica SP5 confocal microscope using an ×20 lens. The movie was quantified by using ImageJ and Ibidi analysis software (http:/ibidi.com/xtproducts/en/Software-and-Image-Analysis/Manual-Image-Analysis/Chemotaxis-and-Migration-Tool).
Fibroblast migration was performed by using the Oris cell migration assay (Platypus Technologies, CMA1.101) according to the manufacturer’s instructions. Briefly, after sealing the Oris Cell Seeding Stoppers in each well, 40 000 cells were seeded in each well and left to adhere for 24 hours. The cell seeding stoppers were then gently removed, the cells were washed twice with phophate-buffered saline, and fresh media was added. Cell migration was then monitored at 37°C for 18 hours by using an Axiovert 135 microscope equipped with a motorized stage that captured one image every 15 minutes. Migration was quantified by measuring the area invaded by the cells by using ImageJ/Fiji software.
Supplemental methods
Details of the reagents, short hairpin RNA (shRNA), cell transduction, confocal microscopy, flow cytometry, phagocytosis assay, western blot quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and cell migration analysis are provided in the supplemental Data available on the Blood Web site.
Results
Human MKL1 deficiency is associated with severe bacterial infections, neutrophil dysfunction, and actin cytoskeletal abnormalities
We report a girl born to second cousin consanguineous parents (Figure 1A) who presented in the first year of life with Pseudomonas septic shock associated with meningitis, malignant otitis externa, and more than 30 cutaneous and subcutaneous abscesses. This followed a primary chickenpox infection that was considered to have a normal course with many lesions that had crusted over by 2 weeks and which did not require hospitalization. Around 2 weeks after the onset of chickenpox, she presented with another pustule in her ear that was initially thought to be a recurrence of varicella-zoster virus. However, she developed skin lesions and became acutely unwell with Pseudomonas septicemia. She was very slow to respond to appropriate antibiotic therapy and received a month of intravenous antibiotics before being discharged from the hospital with oral ciprofloxacin therapy. She recovered and was started on antibiotic prophylaxis and empiric immunoglobulin replacement therapy. Despite this, she continued to have intermittent deep skin infections characterized by purple painful lesions, usually in the peripheries, that responded to oral antibiotics. In the past, she had received 7 days of intravenous antibiotics for possible sepsis at age 2 days, had a history of Pseudomonas ear infections, and failed to thrive. Bacille Calmette-Guérin vaccination, given during the neonatal period, resulted in a large ulcerated abscess that required several months of oral antimycobacterial treatment.
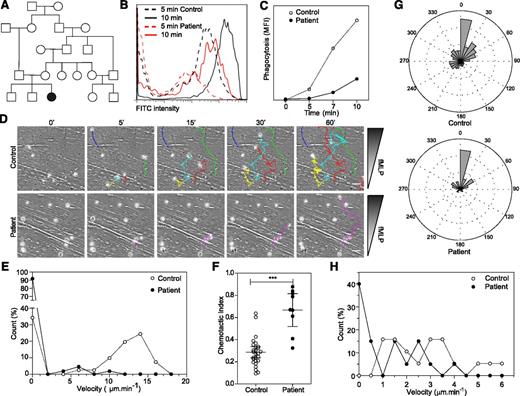
Patient neutrophils display phagocytosis and migration defects. (A) Family tree. (B-C) Fluorescein isothiocyanate (FITC) –positive Escherichia coli uptake by neutrophils analyzed by flow cytometry. Representative data from 2 independent experiments. (D) Migration of control and patient neutrophils in a Dunn chamber in response to an fMLP gradient. Tracks show migration path over time. The number of cells analyzed was 41 in the control and 110 and patient sample in 1 experiment. Scale bar = 10 μm. (E) Frequency distribution of the velocity of patient and control neutrophils. (F) Chemotactic index of the migrating neutrophils. (G) Distribution of instantaneous angle between the gradient and the cell path during migration. (H) Frequency distribution of the velocity of control and patient B lymphoblastoid cell lines migrating in collagen gel. Twenty cells were analyzed in each population in 1 experiment. Data are representative of 2 independent experiments. *** P < .001. MFI, mean fluorescent intensity.
Patient neutrophils display phagocytosis and migration defects. (A) Family tree. (B-C) Fluorescein isothiocyanate (FITC) –positive Escherichia coli uptake by neutrophils analyzed by flow cytometry. Representative data from 2 independent experiments. (D) Migration of control and patient neutrophils in a Dunn chamber in response to an fMLP gradient. Tracks show migration path over time. The number of cells analyzed was 41 in the control and 110 and patient sample in 1 experiment. Scale bar = 10 μm. (E) Frequency distribution of the velocity of patient and control neutrophils. (F) Chemotactic index of the migrating neutrophils. (G) Distribution of instantaneous angle between the gradient and the cell path during migration. (H) Frequency distribution of the velocity of control and patient B lymphoblastoid cell lines migrating in collagen gel. Twenty cells were analyzed in each population in 1 experiment. Data are representative of 2 independent experiments. *** P < .001. MFI, mean fluorescent intensity.
Initial investigations revealed intermittent mild thrombocytopenia (platelets typically ranging from 50 to 150 × 109/L). Complement function, minimum bactericidal level, CD11a and CD18 expression, neutrophil oxidative burst activity, lymphocyte subsets, naive and memory T-cell numbers, and T-cell proliferation to phytohemagglutinin were normal. Her immunoglobulin levels were also unremarkable; vaccine responses were not measured (supplemental Table 1). Only her T-cell proliferation to anti-CD3 antibody was defective, suggesting a specific defect downstream of the T-cell receptor. In view of a clinical suspicion of neutrophil dysfunction, neutrophil phagocytosis was tested by using preopsonized Escherichia coli and was found to be abnormal on two occasions (Figure 1B-C). Because this suggested a defect of cytoskeletal function, neutrophil chemotaxis in response to fMLP was also tested by using a Dunn migration chamber.11 In comparison with healthy donor neutrophils, patient neutrophils showed a severe migratory defect, with >90% of cells unable to migrate. The few cells that migrated did so very slowly (Figure 1D-E and supplemental videos 1-2), although chemotaxis (migration along the chemokine gradient) was preserved (Figure 1F), suggesting retention of functional signal transduction in response to fMLP. In fact, patient cells demonstrated enhanced directional persistence as shown by the chemotactic index (Figure 1F-G and supplemental videos 1-2), suggesting impaired regulation of F-actin remodeling, chemokine signaling, or integrin function.12 An Epstein-Barr virus–immortalized lymphoblastoid cell line from the patient also showed impaired migration toward MIP-3α (Figure 1H). In keeping with an actin cytoskeletal defect, we found reduced F-actin levels in both myeloid and lymphoid cells (Figure 2A-C).
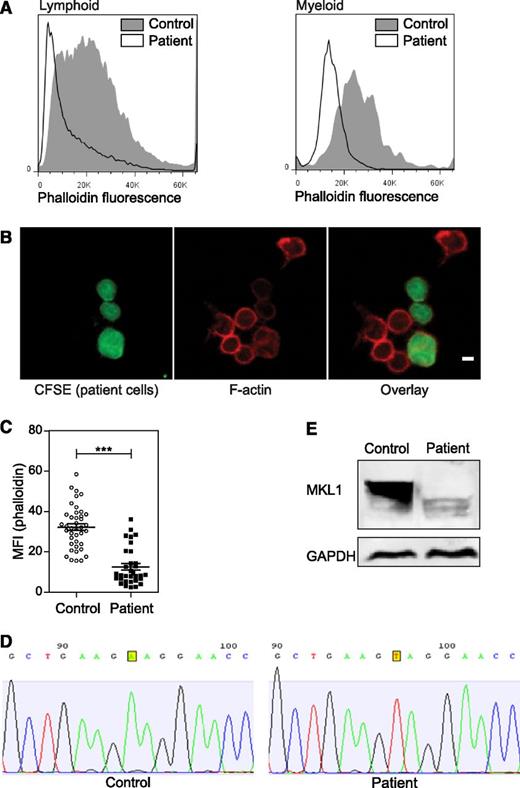
Effect of MKL1 mutation on F-actin. (A) F-actin (Alexa Fluor 647 phalloidin) content in lymphoid and myeloid cells was analyzed by flow cytometry. Patient cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and mixed with unstained control cells before fixation and staining. Representative data from 2 experiments. (B) Confocal images of patient cells (CFSE, green), F-actin (Alexa Fluor 647 phalloidin, red), and mixed with control cells (unstained). Scale bar = 5 μm. Data were analyzed from 1 experiment. (C) Quantification of the phalloidin MFI observed in (B). (D) Sequence analysis of control and patient samples leading to the identification of the MKL1 mutation. (E) MKL1 expression visualized by western blot in control and patient peripheral blood mononuclear cells (PBMCs). ***P < .001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of MKL1 mutation on F-actin. (A) F-actin (Alexa Fluor 647 phalloidin) content in lymphoid and myeloid cells was analyzed by flow cytometry. Patient cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and mixed with unstained control cells before fixation and staining. Representative data from 2 experiments. (B) Confocal images of patient cells (CFSE, green), F-actin (Alexa Fluor 647 phalloidin, red), and mixed with control cells (unstained). Scale bar = 5 μm. Data were analyzed from 1 experiment. (C) Quantification of the phalloidin MFI observed in (B). (D) Sequence analysis of control and patient samples leading to the identification of the MKL1 mutation. (E) MKL1 expression visualized by western blot in control and patient peripheral blood mononuclear cells (PBMCs). ***P < .001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The clinical and cellular phenotype of the patient suggested a novel disorder. Because the patient was born to a consanguineous family, we hypothesized that her disease was caused by a recessive Mendelian mutation, and we used whole exome sequencing to identify it. In the exome data, we found 20 590 single nucleotide variants and small insertions or deletions, including 315 that were very rare (ie, those not seen in the 6,500 National Heart, Lung, and Blood Institute Exomes, 1000 Genomes database [April 2012 data release], or 2,500 exomes analyzed internally using the same bioinformatics pipeline). Of these rare variants, 3 were homozygous (supplemental Table 2), including 1 nonsense variant p.K723X in the MKL1 gene, which is predicted to truncate the 931-amino-acid MKL1 protein. Sanger sequencing confirmed the presence of the mutation (Figure 2D). Western blot of PBMCs confirmed the absence of the full-length MKL1 protein (Figure 2E). A smaller protein band was seen in patient cells, which could represent low levels of truncated MKL1.
MKL1 deficiency impairs neutrophil migration
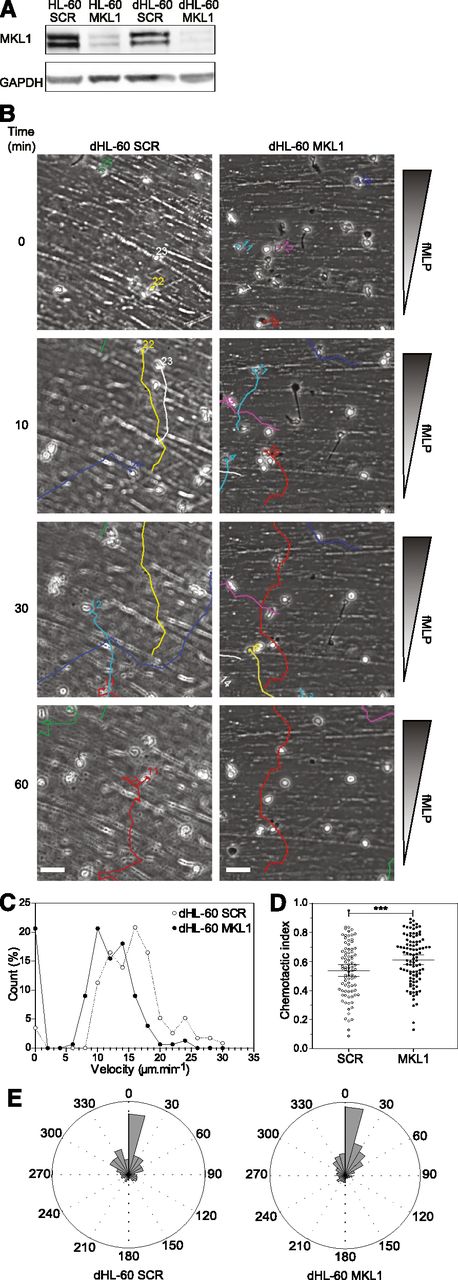
Although MKL1 is known to be important for cytoskeletal regulation, to the best of our knowledge, its role in immune cell function has never been studied. To confirm that impaired neutrophil function resulted from disruption of MKL1 in our patient, we used shRNA lentiviral vectors to specifically silence MKL1 in an HL-60 myeloid cell line (Figure 3A) that can be differentiated to neutrophils (dHL-60 cells). Silencing of MKL1 in HL-60 cells (dHL-60 MKL1) did not alter differentiation potential as reflected by nuclear morphologic change, upregulation of CD11b, and downregulation of CD29/CD49d surface expression (supplemental Figure 1A,C). Surface expression of the fMLP receptor FPR1 was also not affected by MKL1 silencing (supplemental Figure 1B). In spite of this, directional migration in response to fMLP was markedly abrogated, and chemotaxis was associated with enhanced directional persistence (Figure 3B-E and supplemental Videos 3-4). The migratory defect in dHL-60 MKL1 cells therefore exactly mimicked the phenotype of patient neutrophils.
Knockdown of MKL1 in HL-60 neutrophil-like cells mimics patient phenotype. HL-60 cells were differentiated into neutrophil-like cells for 5 days in culture media containing 1.3% dimethylsulfoxide. (A) Western blot of the MKL1 expression levels in undifferentiated (HL-60) or differentiated (dHL-60) cells carrying SCR or the shRNA against MKL1 (MKL1). (B) Migration of SCR and MKL1 neutrophils in a Dunn chamber in response to an fMLP gradient. Tracks show migration path over time. Scale bar = 20 μm. (C) Frequency distribution of the velocity of dHL-60 SCR and MKL1 cells during migration in a Dunn chamber in response to fMLP. The number of cells analyzed was 115 for SCR and 156 for MKL1 cells in 3 independent experiments. (D) Chemotactic index of dHL-60 SCR and dHL-60 MKL1 cells during migration in a Dunn chamber. (E) Instant angle distribution during the migration of SCR and MKL1 dHL-60 cells. ***P < .001.
Knockdown of MKL1 in HL-60 neutrophil-like cells mimics patient phenotype. HL-60 cells were differentiated into neutrophil-like cells for 5 days in culture media containing 1.3% dimethylsulfoxide. (A) Western blot of the MKL1 expression levels in undifferentiated (HL-60) or differentiated (dHL-60) cells carrying SCR or the shRNA against MKL1 (MKL1). (B) Migration of SCR and MKL1 neutrophils in a Dunn chamber in response to an fMLP gradient. Tracks show migration path over time. Scale bar = 20 μm. (C) Frequency distribution of the velocity of dHL-60 SCR and MKL1 cells during migration in a Dunn chamber in response to fMLP. The number of cells analyzed was 115 for SCR and 156 for MKL1 cells in 3 independent experiments. (D) Chemotactic index of dHL-60 SCR and dHL-60 MKL1 cells during migration in a Dunn chamber. (E) Instant angle distribution during the migration of SCR and MKL1 dHL-60 cells. ***P < .001.
MKL1 regulates cytoskeletal functions of DCs
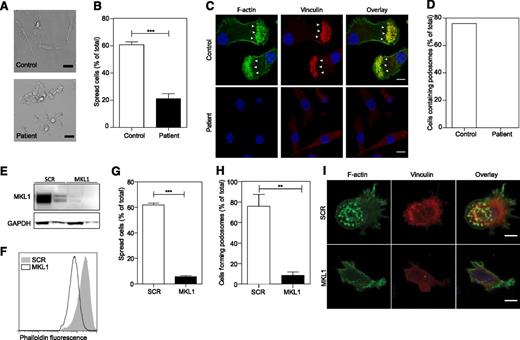
To determine whether MKL1 is required for cytoskeletal regulation in other myeloid lineage immune cells, we generated DCs from patient CD14+ monocytes and analyzed them for cytoskeletal morphology and adhesion. We found that patient MKL1-deficient DCs were morphologically distinct from control DCs and showed reduced spreading on fibronectin-coated coverslips (Figure 4A-B), a marked reduction in total F-actin staining, and a complete absence of podosomes (Figure 4C-D). Targeted shRNA silencing of MKL1 in DCs derived from the THP1 cell line (Figure 4E) recapitulated these findings resulting in marked depletion of F-actin, poor adhesion to fibronectin, reduced spreading, and defective podosome assembly (Figure 4F-I).
Patient PBMC-derived DCs have morphologic abnormalities and severely reduced F-actin content. DCs were left to adhere to fibronectin-coated glass coverslips for 4 hours and were then fixed in 4% paraformaldehyde. (A) Phase contrast imaging shows poor spreading of patient DCs compared with controls. Scale bar = 20 μm. (B) Quantification of the percent of cells that had spread. (C-D) Cells were stained for DNA (4,6 diamidino-2-phenylindole, blue), F-actin (Alexa Fluor 488 phalloidin, green), and vinculin (red), and images were acquired by confocal microscopy. Podosome structures were clearly present in control cells (F-actin cores surrounded by vinculin in 22 of 29 cells; white arrows) but completely absent in patient cells (0 of 121 cells). Data were acquired in a single experiment. Scale bar = 10 μm. (E) Western blot of MKL1 in THP1 cells expressing SCR (THP1 SCR) and against MKL1 (THP1 MKL1). (F) F-actin content in THP1 SCR and THP1 MKL1 DCs analyzed by flow cytometry. (G) Quantification of the spreading of THP1 SCR and THP1 MKL1 DCs. (H-I) Confocal images of F-actin- and vinculin-stained cells demonstrate the lack of podosomes in THP1 MKL1 DCs. Scale bar = 10 μm. THP1 MKL1 DC data were acquired in 3 independent experiments. **P < 0.01; ***P < .001.
Patient PBMC-derived DCs have morphologic abnormalities and severely reduced F-actin content. DCs were left to adhere to fibronectin-coated glass coverslips for 4 hours and were then fixed in 4% paraformaldehyde. (A) Phase contrast imaging shows poor spreading of patient DCs compared with controls. Scale bar = 20 μm. (B) Quantification of the percent of cells that had spread. (C-D) Cells were stained for DNA (4,6 diamidino-2-phenylindole, blue), F-actin (Alexa Fluor 488 phalloidin, green), and vinculin (red), and images were acquired by confocal microscopy. Podosome structures were clearly present in control cells (F-actin cores surrounded by vinculin in 22 of 29 cells; white arrows) but completely absent in patient cells (0 of 121 cells). Data were acquired in a single experiment. Scale bar = 10 μm. (E) Western blot of MKL1 in THP1 cells expressing SCR (THP1 SCR) and against MKL1 (THP1 MKL1). (F) F-actin content in THP1 SCR and THP1 MKL1 DCs analyzed by flow cytometry. (G) Quantification of the spreading of THP1 SCR and THP1 MKL1 DCs. (H-I) Confocal images of F-actin- and vinculin-stained cells demonstrate the lack of podosomes in THP1 MKL1 DCs. Scale bar = 10 μm. THP1 MKL1 DC data were acquired in 3 independent experiments. **P < 0.01; ***P < .001.
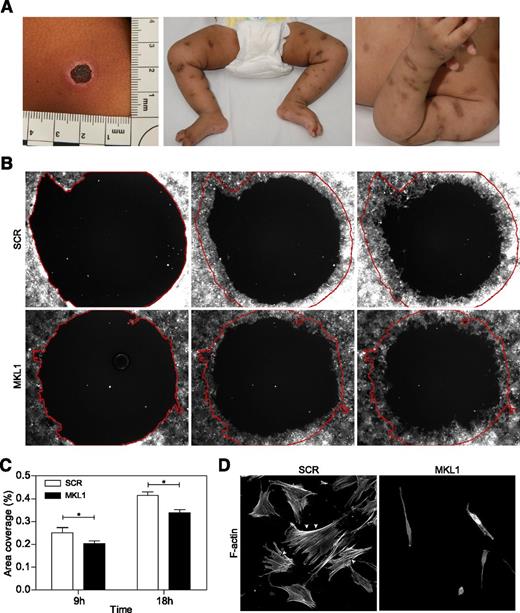
MKL1 alters fibroblast migration
Significant and abnormal scarring was a prominent feature of our patient’s response to skin infections (Figure 5A), which suggested a possible additional defect of fibroblast function. To test this (in the absence of primary fibroblasts from the patient), we examined migration of normal primary fibroblasts following lentiviral transduction with scrambled or MKL1-targeting shRNA (transduction efficiencies of 97% and 81%, respectively). In a modified wound healing assay, only fibroblasts expressing MKL1 shRNA demonstrated impaired migration in vitro over 18 hours (Figure 5B-C). This was associated with dramatically altered cell shape and loss of cortical actin and stress fibers when compared with cells expressing scrambled shRNA (SCR) (Figure 5D). These findings indicate an important role for MKL1-mediated cytoskeletal regulation in nonhematopoietic cells.
Knockdown of MKL1 alters fibroblast morphology and migration. (A) Clinical photos showing skin scarring and hyperpigmentation after treating skin abscesses with antibiotics. (B) Migration of fibroblasts expressing SCR and MKL1 shRNA at 0, 9, and 18 hours. (C) Quantification of the area of migration observed in (B). (D) Confocal images of fibroblasts expressing SCR and MKL1 shRNA. Cells were stained with Alexa Fluor 647 phalloidin. White arrows indicate stress fibers. *P < .05.
Knockdown of MKL1 alters fibroblast morphology and migration. (A) Clinical photos showing skin scarring and hyperpigmentation after treating skin abscesses with antibiotics. (B) Migration of fibroblasts expressing SCR and MKL1 shRNA at 0, 9, and 18 hours. (C) Quantification of the area of migration observed in (B). (D) Confocal images of fibroblasts expressing SCR and MKL1 shRNA. Cells were stained with Alexa Fluor 647 phalloidin. White arrows indicate stress fibers. *P < .05.
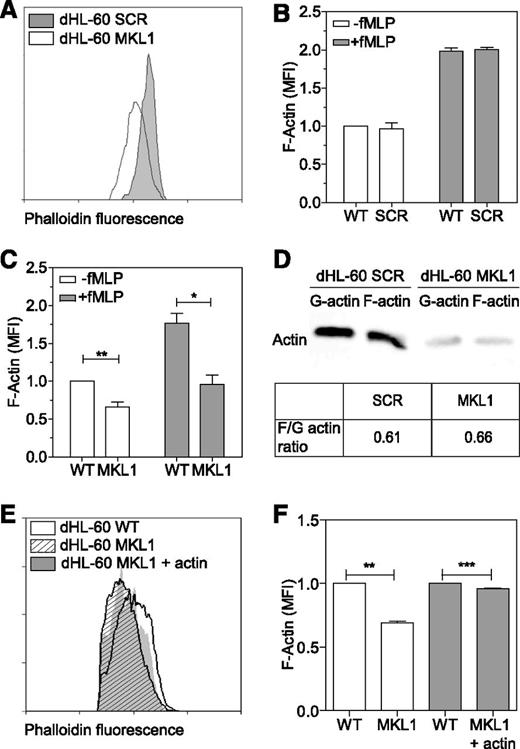
MKL1 regulates neutrophil actin cytoskeleton dynamics
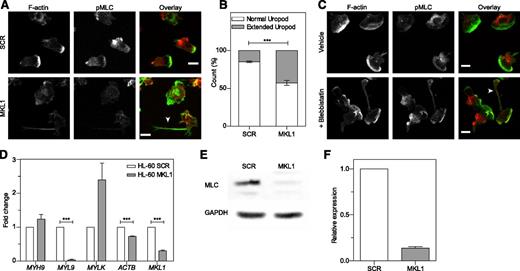
As seen in patient cells, dHL-60 MKL1 cells displayed reduced F-actin content (Figure 6A-C). Quantification of the F-actin and G-actin cellular fractions by western blot showed that SCR and dHL-60 MKL1 had a similar G-actin:F-actin ratio but that MKL1-deficient cells displayed a reduction in both G-actin and F-actin content (Figure 6D). To test whether loss of G-actin is the cause of cytoskeletal dysfunction in MKL1 deficiency, we reconstituted G-actin expression by transducing dHL-60 MKL1 cells with a β-actin-mCherry lentivirus vector. Although G-actin reconstitution partially restored F-actin content, a significant reduction in F-actin levels persisted (Figure 6E-F), suggesting additional defects of actin regulation in cells lacking MKL1. By using cytoskeletal-specific PCR arrays, we observed alteration in the expression levels of multiple regulators of the actin cytoskeleton in dHL-60 MKL1 cells; CDC42BPA, CTTN, and FNBP1L were significantly downregulated whereas expression of a number of other actin-associated genes were upregulated in the absence of MKL1 (Table 1 and supplemental Table 3). A notable feature of dHL-60 MKL1 cells was an abnormally elongated uropod, suggesting impaired retraction during migration. When uniformly stimulated with fMLP, 43% (± 5%) of MKL1-silenced dHL-60 cells displayed this morphology compared with 15% (± 1.5%) of control cells (Figure 7A-B). Because uropod retraction is regulated by the myosin II protein complex,13 we reasoned that MKL1 deficiency could impair neutrophil migration through effects on myosin II. As expected, disruption of myosin II interaction with F-actin using blebbistatin produced impaired uropod retraction similar14 to that observed in dHL-60 MKL1 cells (Figure 7C). Furthermore, expression of regulatory myosin light chain (MYL9/MLC), which is a component of myosin II complex, was dramatically inhibited in dHL-60 MKL1 cells (Figure 7D-F).
MKL1-deficient dHL-60 cells show lower content in F-actin. (A) Analysis of the F-actin content in dHL-60 MKL1 cells by flow cytometry. dHL-60 wild-type (WT) cells and dHL-60 cells carrying a scrambled or anti-MKL1 shRNA and coexpressing green fluorescent protein were mixed before fixation and staining for F-actin with Alexa Fluor 647 phalloidin. For clarity, only SCR and MKL1 populations are displayed on the flow cytometry graph. (B-C) Evaluation of the F-actin content in dHL-60 cells after stimulation with 100 nM fMLP for 1 minute in SCR and MKL1 populations, respectively. Data from 3 independent experiments were analyzed. (D) G-actin and F-actin content in dHL-60 SCR and MKL1 cells. (E-F) Analysis of the F-actin content in dHL-60 cells after stimulation with 100 nM fMLP for 1 minute in WT, MKL1, and MKL1 cells expressing mCherry G-actin. *P < .05; **P < .01; ***P < .001.
MKL1-deficient dHL-60 cells show lower content in F-actin. (A) Analysis of the F-actin content in dHL-60 MKL1 cells by flow cytometry. dHL-60 wild-type (WT) cells and dHL-60 cells carrying a scrambled or anti-MKL1 shRNA and coexpressing green fluorescent protein were mixed before fixation and staining for F-actin with Alexa Fluor 647 phalloidin. For clarity, only SCR and MKL1 populations are displayed on the flow cytometry graph. (B-C) Evaluation of the F-actin content in dHL-60 cells after stimulation with 100 nM fMLP for 1 minute in SCR and MKL1 populations, respectively. Data from 3 independent experiments were analyzed. (D) G-actin and F-actin content in dHL-60 SCR and MKL1 cells. (E-F) Analysis of the F-actin content in dHL-60 cells after stimulation with 100 nM fMLP for 1 minute in WT, MKL1, and MKL1 cells expressing mCherry G-actin. *P < .05; **P < .01; ***P < .001.
Differential expression of actin cytoskeleton genes in MKL1-deficient cells
| Gene symbol . | Fold change . |
|---|---|
| Upregulated genes | |
| ARHGEF11 | 3.2422 |
| ARPC1B | 1.7815 |
| ARPC4 | 1.6004 |
| CDC42EP2 | 1.639 |
| CDC42EP3 | 1.7405 |
| CRK | 1.7697 |
| CYFIP2 | 1.6496 |
| FSCN2 | 2.6368 |
| LIMK1 | 1.6485 |
| PFN2 | 1.6517 |
| RAC1 | 1.8577 |
| WAS | 1.8735 |
| WASL | 1.5468 |
| Downregulated genes | |
| CDC42BPA | −2.9896 |
| CTTN | −6.1696 |
| FNBP1L | −1.622 |
| ACTB | −1.5001 |
| Gene symbol . | Fold change . |
|---|---|
| Upregulated genes | |
| ARHGEF11 | 3.2422 |
| ARPC1B | 1.7815 |
| ARPC4 | 1.6004 |
| CDC42EP2 | 1.639 |
| CDC42EP3 | 1.7405 |
| CRK | 1.7697 |
| CYFIP2 | 1.6496 |
| FSCN2 | 2.6368 |
| LIMK1 | 1.6485 |
| PFN2 | 1.6517 |
| RAC1 | 1.8577 |
| WAS | 1.8735 |
| WASL | 1.5468 |
| Downregulated genes | |
| CDC42BPA | −2.9896 |
| CTTN | −6.1696 |
| FNBP1L | −1.622 |
| ACTB | −1.5001 |
MKL1-deficient dHL-60 cells show impaired uropod retraction associated with downregulation of MYL9. (A) Knockdown of MKL1 induces a uropod retraction defect. dHL-60 cells were left to adhere to fibronectin-coated glass coverslips for 20 minutes, washed, then uniformly stimulated with fMLP for 2 minutes and fixed in 4% paraformaldehyde. Cells were then stained for F-actin and phosphorylation of MLC (pMLC). The dHL-60 MKL1 F-actin staining intensity was increased threefold in the merged (overlay) image. Images were taken with an ×40 lens. White arrow indicates extended uropod. (B) Quantification of the frequency of uropod extension in dHL-60 MKL1 cells observed in (A). More than 250 cells were analyzed in each condition in 2 independent experiments. (C) Inhibition of myosin II interaction with F-actin using blebbistatin (100 µM) induced a uropod retraction defect in dHL-60 WT cells. Images were taken with a ×63 lens. Scale bars = 10 μm. (D) mRNA expression in HL-60 SCR and MKL1 cells analyzed by qRT-PCR. (E-F) Myosin light chain expression was evaluated by western blot. ***P < .001.
MKL1-deficient dHL-60 cells show impaired uropod retraction associated with downregulation of MYL9. (A) Knockdown of MKL1 induces a uropod retraction defect. dHL-60 cells were left to adhere to fibronectin-coated glass coverslips for 20 minutes, washed, then uniformly stimulated with fMLP for 2 minutes and fixed in 4% paraformaldehyde. Cells were then stained for F-actin and phosphorylation of MLC (pMLC). The dHL-60 MKL1 F-actin staining intensity was increased threefold in the merged (overlay) image. Images were taken with an ×40 lens. White arrow indicates extended uropod. (B) Quantification of the frequency of uropod extension in dHL-60 MKL1 cells observed in (A). More than 250 cells were analyzed in each condition in 2 independent experiments. (C) Inhibition of myosin II interaction with F-actin using blebbistatin (100 µM) induced a uropod retraction defect in dHL-60 WT cells. Images were taken with a ×63 lens. Scale bars = 10 μm. (D) mRNA expression in HL-60 SCR and MKL1 cells analyzed by qRT-PCR. (E-F) Myosin light chain expression was evaluated by western blot. ***P < .001.
Discussion
MKL1 was first identified in association with acute megakaryoblastic leukemia, in which it is a component of a fusion protein generated by chromosomal translocation.15 Subsequent investigation of MKL1 function in hematopoietic cells has focused on megakaryocytes, and MKL1 deficiency in mice has been shown to result in cytoskeletal defects in this cell type disrupting differentiation, migration, and proplatelet formation.9,10,16 Our study is the first to describe human MKL1 deficiency, and it demonstrates that although mild thrombocytopenia is associated with the condition, the major phenotype appears to relate to immunodeficiency.
Through direct interaction with transcription factor SRF, MKL1 regulates the translation of cytoskeletal genes17 in multiple cell types to control cellular processes including migration, adhesion, and differentiation.9,18-20 In keeping with this, we demonstrate here that MKL1 plays a nonredundant role in maintaining normal levels of F-actin in cells with both lymphoid and myeloid lineage. Because the infection phenotype of our patient was prominent bacterial susceptibility, we initially studied the impact of MKL1 deficiency on neutrophils. In the absence of MKL1 activity, the neutrophil actin cytoskeleton is severely perturbed as shown by defects in phagocytosis and migration. Our data suggest that the mechanisms of cytoskeletal dysfunction in MKL1-deficient cells are multifactorial. A significant component is reduced expression of G-actin, which we observed at both RNA and protein levels. However, forced reconstitution of G-actin by using lentivectors did not fully restore intracellular F-actin levels, which suggests that other MKL1-dependent cytoskeletal regulators are critical for normal F-actin assembly. By using qPCR arrays, we found that multiple actin-associated genes were differentially expressed, which supports a complex role for MKL1 in cytoskeletal dynamics. Somewhat surprisingly, only a minority of actin-associated genes were downregulated (3 of 18), although this group included cortactin (CTTN), CDC42BPA, and FNBP1L, all of which promote F-actin assembly through the CDC42/WASp/Arp2/3 pathway in hematopoietic cells.21-24 In keeping with the role of MKL1 as an SRF activator, our findings are reminiscent of the SRF-deficient mouse model in which neutrophils were shown to demonstrate reduced levels of F-actin associated with impaired polarization, migration, and recruitment to areas of inflammation.25 In both MKL1 and SRF deficiency, neutrophil numbers appear to be unaffected, which suggests that despite causing intrinsic cytoskeletal defects, these factors do not significantly impair development or release of neutrophils from the bone marrow.
Neutrophils classically migrate in an amoeboid fashion, characterized by lamellipodial extension and subsequent uropod retraction, for which the family of small GTPases, nonmuscular myosin II, and integrin trafficking are critical regulators.25-28 A prominent feature of MKL1 deficiency in neutrophil-like dHL-60 cells is abnormal uropod extension, suggesting a defect of either myosin complex activity that supports uropod retraction or altered CD11b expression or activation at the cell surface. The uropod phenotype of MKL1-deficient neutrophils is mimicked by chemical inhibition of the myosin complex function. It was previously shown, in CD34+ progenitor cells,9 that loss of MKL1 results in significantly reduced expression of MYL9, a component of the myosin II complex. Subsequent functional analysis demonstrated that MYL9 expression is a key cytoskeletal regulator for both platelet formation and megakaryocyte migration.9 We observed reduced expression of myosin light chain in dHL-60 MKL1 cells, which is in keeping with uropod dysfunction and suggests that MKL1-dependent MYL9 expression is involved in cytoskeletal regulation during neutrophil migration. As seen in the SRF−/− mouse model, MKL1-deficient neutrophils overexpressed CD11b, which may be the result of impaired integrin trafficking.25 In SRF−/− mice, CD11b activation is impaired despite overexpression. Further investigation is required to determine the contribution of abnormal CD11b function to cytoskeletal dysfunction in MKL1 deficiency.
Given the role of MKL1 in megakaryocyte function, we were surprised that the observed thrombocytopenia in our patient was mild and did not lead to a clinically relevant bleeding disorder. This is unlikely to relate to residual expression of a small amount of truncated protein in our patient cells, because it did not appear to preserve significant cytoskeletal function as evidenced by almost complete loss of neutrophil migration and DC podosome assembly. The position of the truncation would be predicted to preserve the B1 basic box and glutamine-rich domains that mediate interaction with SRF4 but disrupt the C-terminal transactivation domain of MKL1 that confers transcriptional activity. This truncation has been modeled in vitro and has been demonstrated to retain nuclear localization but completely abrogate MKL1 activity, which is in keeping with our findings.29 It is possible that the mild effects on platelet production may relate to compensation by MKL2 because mkl1/mkl2 double-knockout mice have a significantly more pronounced platelet deficiency.10
In addition to severe effects on neutrophils and mild effects on platelets, we show that MKL1 is important for cytoskeletal regulation in DCs with myeloid lineage and in lymphoid cells. The clinical phenotype observed may reflect the hierarchical importance of neutrophils for antibacterial defenses, as seen in the CD18 integrin deficiency leukocyte adhesion defect, which presents as a neutrophil disorder, although broader cellular effects exist, which reflect the importance of CD18 in many hematopoietic cell functions.30-32 We also observed that MKL1 deficiency impaired fibroblast migration in vitro. This is consistent with the abnormal wound healing and scarring following superficial skin infections observed in our patient and with previous reports of dysregulated fibroblast migration in vitro when MKL1/SRF function is manipulated genetically or chemically.33 However, it is surprising that other tissues do not appear to be grossly affected, because MKL1 is widely expressed, and deficient mice exhibit partial embryonic lethality with myocardial cell necrosis. Other extrahematopoietic manifestations of human MKL1 deficiency may therefore manifest with increasing age.
Taken together, these data demonstrate a nonredundant role for MKL1 in regulating the actin cytoskeleton in human immune cells and fibroblasts. Our findings in neutrophils, DCs, and B cells suggest a wide impact for MKL1 deficiency on both myeloid and lymphoid immune cell function that would predict complex multilineage immunodeficiency. The clinical phenotype of our patient to date has been dominated by bacterial infections characteristic of neutrophil dysfunction. We do not have evidence of clinically manifesting T- or B-cell defects, although these may be masked by treatment with regular immunoglobulin infusion and prophylactic antibiotics. The full clinical and immunologic phenotype of human MKL1 deficiency will become apparent as additional cases are described. Patients suffering from severe bacterial infections associated with neutrophil migration defects should be screened for possible mutations in the MKL1 gene.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Higher Education Funding Council for England (S.O.B.), The Wellcome Trust (090233/Z/09/Z; A.J.T., D.M., and G.B.), Great Ormond Street Hospital Children’s Charity (K.N.), Child Health Research Appeal (D.M.), University College London Grand Challenge Studentship (J.R.), and the National Institute for Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre (K.G.). S.N. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science (095198/Z/10/Z) and is supported by European Research Council starting grant 260477, EU FP7 collaborative grant 261441 (PEVNET project), and the NIHR Cambridge Biomedical Research Centre. A.J.T. is a Wellcome Trust Principal Fellow.
Authorship
Contribution: J.R. and D.M. designed and performed experiments, analyzed data, and wrote the manuscript; H.L.Z., V.P., K.N., F.S., J.C., and D.M. performed experiments and analyzed data; G.C., G.B., A.J.T., D.M., and K.G. designed experiments; C.C. and S.H. referred patients; and S.N., A.J.T., and S.O.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian J. Thrasher, Institute of Child Health, University College London, 30 Guilford St, London WC1N 1EH, United Kingdom; e-mail: a.thrasher@ucl.ac.uk.
References
Author notes
J.R. and D.M. contributed equally to this work.
S.N., A.J.T., and S.O.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal