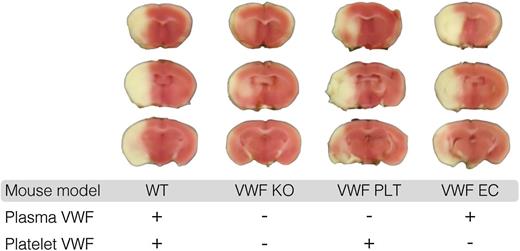
In this issue of Blood, Verhenne et al present data showing the role of platelet-derived von Willebrand factor (VWF) in mediating ischemic stroke injury using a murine model.1 They created mice with either endothelial VWF or platelet-derived VWF and examined each phenotype for bleeding and thrombosis. Their intriguing findings were that mice lacking platelet-derived VWF, but with adequate endothelial VWF stores, demonstrated normal hemostasis in a tail bleeding model and normal carotid artery thrombosis. Mice with only platelet-derived VWF had defective hemostasis and defective carotid artery thrombosis, but experienced significant cerebral infarction using a stroke model with middle cerebral artery occlusion (see figure). In contrast, minimal infarcts were seen for VWF-deficient mice. These data suggest that platelet-derived VWF plays a specific role in stroke pathology.
Platelet-derived VWF mediates ischemic stroke injury. This figure shows representative coronal brain sections 24 hours after induction of transient middle cerebral artery occlusion in wild-type mice (WT), mice completely lacking VWF (VWF KO), mice expressing VWF only in platelets (VWF PLT), and mice expressing VWF only in endothelial cells (VWF EC). White indicates areas of infarct; pink shows unaffected brain tissue. This figure has been modified from Figure 4A in the article by Verhenne et al that begins on page 1715. Professional illustration by Luk Cox, Somersault18:24.
Platelet-derived VWF mediates ischemic stroke injury. This figure shows representative coronal brain sections 24 hours after induction of transient middle cerebral artery occlusion in wild-type mice (WT), mice completely lacking VWF (VWF KO), mice expressing VWF only in platelets (VWF PLT), and mice expressing VWF only in endothelial cells (VWF EC). White indicates areas of infarct; pink shows unaffected brain tissue. This figure has been modified from Figure 4A in the article by Verhenne et al that begins on page 1715. Professional illustration by Luk Cox, Somersault18:24.
VWF is synthesized in both endothelial cells and megakaryocytes. Release of VWF from endothelial cells provides a supply of plasma VWF to participate in coagulation, fulfilling VWF’s purpose as a carrier protein for factor VIII and as a link between exposed collagen and platelets via binding sites for various vascular collagens as well as platelet glycoprotein Ib (GPIb). VWF is also stored in platelet α granules for potential release at sites of injury. The relative importance of these 2 pools of VWF, however, has not been well studied, and based on current data, there may be significant differences in their roles. Supporting evidence for the lack of necessity for platelet-derived VWF in routine hemostasis and thrombosis is provided by Kanaji et al,2 who also demonstrated that endothelial VWF was sufficient, although in their model, platelet-derived VWF did provide some benefit as well.
These data raise the question of what makes platelet-derived VWF so special, and what characteristics impart this stroke-specific phenotype? Platelet-derived VWF has a different glycosylation profile, different affinities for platelet surface receptors, and different multimer distribution, with preferential expression of high molecular weight multimers. Differential glycosylation of platelet-derived VWF may explain many of these findings.3 The pathological consequences of ultralarge VWF multimers are best illustrated in patients with thrombotic thrombocytopenic purpura where a microangiopathic anemia and thrombocytopenia result in part from lack of ADAMTS13 cleavage of these ultralarge multimers and subsequent activation of the alternate complement pathway.4
Platelet binding to VWF can also occur via a binding site on VWF for platelet GPIIbIIIa, and abrogation of that interaction results in decreased time to vessel occlusion in a ferric chloride model.5 However, GPIb appears to be the major mechanism for platelet-VWF interactions in general and for platelet-derived VWF pathology in stroke specifically. Verhenne et al use an anti-GPIbα antibody in their stroke model and show a corresponding reduction in the effect of platelet-derived VWF, as would be expected if GPIb is the major driver in this interaction.1
Platelets themselves have been implicated in stroke, with aspirin playing a key role in treatment and prevention.6 The paradigm in which antiplatelet agents are used, however, has generally been that of preventing platelet aggregation rather than preventing release of prothrombotic VWF. Mice deficient in VWF present with decreased infarct size and less neurologic changes compared with WT mice,7 and humans with von Willebrand disease have a reduced prevalence of stroke and other arterial thromboses.8 Although elevated VWF levels have clearly been associated with arterial thrombosis and stroke, there is also some evidence that ADAMTS13 plays a role, with lower ADAMTS13 levels corresponding to higher stroke risk.9 The association between stroke and ADAMTS13 could be explained by the increase in high-molecular-weight VWF multimers seen in ADAMTS13 deficiency.
The data on platelet-derived VWF and stroke are somewhat limited in terms of the models used. Tail bleeding times, although routinely used to assess hemostasis in mouse models, do not necessarily readily correlate to human hemostasis. Ferric chloride models, although again in routine use, present a decidedly nonphysiologic challenge. Stroke can occur through multiple mechanisms, with varying degrees of dependence on coagulation factors, platelets, and platelet-derived VWF. The experiments described by Verhenne et al, however, do document a clear role for platelet-derived VWF that is specific to ischemic stroke injury.
The insights provided by Verhenne et al raise more questions than they answer. Mice are certainly not humans, and therefore their model requires further study to understand whether or not this pathophysiology is replicated beyond the murine setting. Human and murine VWFs are also not identical. All strokes are not created equal, and platelet-derived VWF may not be relevant in all settings. However, these data do provide an intriguing base for further study of platelet-derived VWF and lend credence to its importance in particular aspects of hemostasis. Plasma VWF has received much attention, in part due to the fact that it is relatively easy to measure. It is only fair that platelet-derived VWF enjoy its role in the spotlight, and perhaps this will yield improved understanding of hemostasis and improved therapies for disease in which platelet-derived VWF plays a key role.
Conflict-of-interest disclosure: V.H.F. has served as a consultant for Baxter.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal