Key Points
Platelet-derived VWF alone mediates full ischemic stroke injury in mice via a GPIb-dependent mechanism.
Platelet-derived VWF does not significantly contribute to normal thrombosis and hemostasis in mice.
Abstract
Von Willebrand factor (VWF) is a key hemostatic protein synthesized in both endothelial cells and megakaryocytes. Megakaryocyte-derived VWF is stored in α-granules of platelets and is enriched in hyperactive “ultra-large” VWF multimers. To elucidate the specific contribution of platelet VWF in hemostasis and thrombosis, we performed crossed bone marrow transplantations between C57BL/6J and Vwf−/− mice to generate chimeric mice. Chimeric mice specifically lacking platelet VWF showed normal tail bleeding and carotid artery thrombosis, similar to wild-type mice. Chimeric mice with VWF present only in platelets were not able to support normal thrombosis and hemostasis. However, using a mouse model of transient middle cerebral artery occlusion, we observed that cerebral infarct sizes and fibrin(ogen) deposition in chimeric mice with only platelet VWF were significantly increased compared with Vwf−/− mice (P < .01). Blocking of the platelet VWF-glycoprotein (GP)Ib interaction abrogated this platelet VWF-mediated injury. These data suggest that whereas platelet-derived VWF does not play a crucial role in hemostasis and arterial thrombosis, it aggravates thrombo-inflammatory diseases such as stroke via a GPIb-dependent mechanism.
Introduction
Von Willebrand factor (VWF) is an adhesive multimeric glycoprotein that is crucial for normal hemostasis. The best-known functions of VWF are facilitating platelet adhesion at sites of vascular injury and protecting clotting factor VIII against early degradation in the circulation. Consequently, absence or dysfunction of VWF results in bleeding symptoms, as observed in patients with von Willebrand disease.1 Intriguingly, it has become clear that VWF is also implicated in various nonhemostatic processes, such as tumor metastasis, inflammation, and angiogenesis.2 In particular, the pathophysiological role of VWF in ischemic stroke has recently gained increased attention.3-7
Biosynthesis of VWF is restricted to endothelial cells (ECs) and megakaryocytes. Endothelial VWF is constitutively secreted into plasma and subendothelium, or stored as “ultra-large” (UL)-VWF multimers in Weibel-Palade bodies. VWF produced in megakaryocytes ends up as UL-VWF in the α-granules of platelets. VWF present in endothelial and platelet storage organelles is secreted in a regulated process in response to stimulation by secretagogues. Platelets contain a significant amount of VWF, accounting for 15% to 20% of the total amount of circulating VWF. Interestingly, an increasing body of evidence shows that platelet VWF differs in various biochemical aspects from endothelial VWF.8 Williams et al9 showed that platelet VWF binds more efficiently to glycoprotein (GP)IIb/IIIa and heparin in comparison with plasma VWF, whereas, it is less capable of binding to GPIb. Divergent glycosylation profiles could explain these differences. Indeed, platelet VWF exists as a distinct glycoform, characterized by a significantly reduced degree of N-linked sialylation and by the absence of expression of AB blood group determinants.8,10 This differential glycosylation also renders platelet VWF more resistant to ADAMTS13 proteolysis.10 With platelet VWF being enriched in UL-VWF, this resistance to ADAMTS13 cleavage could become particularly relevant in settings where local accumulation of UL-VWF is detrimental, such as cerebral or myocardial ischemia/reperfusion injury.6,11,12 Given variable platelet VWF antigen and activity levels between individuals, it is important to understand the biological activity of this specific pool of VWF. Only a few studies have tried to dissect the different (patho)physiological roles of platelet and plasma VWF. Although experimental evidence shows that platelet VWF indeed contributes to initial platelet adhesion to collagen at sites of vascular injury13-15 in vivo data on the role of platelet VWF in thrombosis is limited. The role of VWF from platelets in ischemic stroke has never been investigated. In the present study, we found that plasma VWF, but not platelet VWF is needed for normal hemostasis and carotid artery thrombus formation, whereas platelet VWF by itself is able to mediate ischemic brain injury via a GPIb-dependent mechanism, shedding new light on the role of platelet VWF in thrombo-inflammatory disease.
Methods
Mice
Vwf−/− (VWF knockout [KO])16 and littermate wild-type (WT) C57BL/6J mice were used. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the KU Leuven (Belgium).
Bone marrow transplantation
Bone marrow cells were collected from the femur and tibia of 6- to 10-week-old donor mice. The mononuclear cell (MNC) population was isolated using Ficoll gradient centrifugation (Ficoll-Paque Premium 1.084; GE Healthcare, Waukesha, IL). The 6- to 8-week-old recipient VWF KO and WT mice were conditioned for cellular transplantation with a single lethal dose of 1100 cGy total body irradiation using a dual linear energy accelerator (Clinac DHX, 6MV; Varian Medical Systems, CA). Twenty-four hours after irradiation 10 × 106 MNC, dissolved in 250 μL sterile phosphate-buffered saline, were infused by intravenous injection in irradiated recipient mice. Three to four weeks after transplantation, full chimerism was confirmed via flow cytometry.
Blood collection
Animals were anesthetized using 5% isoflurane (Nicholas Piramal Limited, London, UK) in 100% O2. Blood was collected by retro-orbital puncture on 0.5 M EDTA (1 volume to 40 volumes of blood) or 3.8% trisodium citrate (1 volume to 6 volumes of blood). EDTA-treated blood was used to determine platelet counts using the Hemavet 950FS Multi-species Hematology system (Drew Scientific, Oxford, CT). Platelet-rich plasma (PRP) was prepared from citrated blood by centrifugation at 325 g for 5 minutes at room temperature and immediately used for flow cytometric analysis. Platelet-poor plasma was prepared from citrated blood by centrifugation at 4300 g for 6 minutes at 4°C and stored at −20°C for further analysis.
Flow cytometry
At 3 to 4 weeks after transplantation, citrated PRP was prepared from chimeric and control mice. After fixation, permeabilized platelets were incubated with a primary rabbit anti-human VWF antibody (1:100; Dako, Glostrup, Denmark) and a secondary fluorescein isothiocyanate-conjugated swine anti-rabbit antibody (1:40; Dako), as described in the supplemental Methods on the Blood web site. To quantify the relative amount of VWF-positive platelets, a standard curve (0%, 25%, 50%, 75%, and 100% VWF-positive platelets) was made by combining PRP from WT and VWF KO mice. Results were expressed as percentage of WT values. To identify the platelet population, platelets were stained with rat anti-mouse CD41 antibody conjugated with phycoerythrin (1:50; eBioscience, San Diego, CA). Expression of CD41 and intracellular platelet VWF was determined using a Beckman Coulter EPICS XL-MCL flow cytometer and analyzed with the WinMDI 2.9 free FACS analysis software.
VWF and FVIII analysis
VWF antigen levels in plasma were determined using an in-house developed enzyme-linked immunosorbent assay. A detailed description of this assay is provided in the supplemental Methods. Factor VIII (FVIII) activity (FVIII:C) in mouse plasma was determined using the COATEST SP4 FVIII (Chromogenix, Molndal, Sweden), according to the manufacturer’s protocol. Pooled plasma from 10 to 20 WT mice was used as a reference (100%) and results were expressed as percentage of WT values.
Tail clip bleeding time assay
Tail clip bleeding times were assessed as described.17 At 5 to 6 weeks after transplantation, mice were anesthetized with intraperitoneal injection of pentobarbital (80 μg/g; Nembutal, Ceva Santé Animale, Brussels, Belgium). Immediately after removing 5 mm of the tail tip using a surgical scalpel, the tail was immersed in 0.9% NaCl prewarmed at 37°C. The time until blood loss ceased was monitored. The experiment was stopped when bleeding did not stop within 600 seconds.
FeCl3-induced carotid artery thrombosis model
At 8 to 10 weeks after transplantation, mice were anesthetized using 2.5% isoflurane in 100% O2. The right carotid artery was subsequently exposed and a Doppler flow probe (Transonic TS420 perivascular flowmeter module; AD Instruments, Oxford, UK) was positioned around the artery to monitor blood flow via a laser Doppler perfusion monitor (PeriFlux System 5000; Perimed AB, Järfälla-Stockholm, Sweden) coupled to a PowerLab 8/35 data acquisition unit (AD Instruments) using LabChart v8.0.5 software (AD Instruments). Arterial thrombosis was induced by applying a piece of Whatman filter paper (1 × 1 mm; GE Healthcare) saturated in 12% FeCl3 solution, for 3 minutes upstream of the flow probe. The time to occlusion was recorded. If the time to occlusion exceeded 50 minutes, the experiment was ended.
Transient middle cerebral artery occlusion
Focal cerebral ischemia was induced 8 to 10 weeks after transplantation by 60 minutes transient middle cerebral artery occlusion, as described.5 To block the VWF-GPIb interaction, 100 μg rat anti-mouse GPIbα (p0p/B) Fab18 or rat IgG Fab control was injected intravenously 5 minutes after inducing reperfusion of the right middle cerebral artery. All stroke experiments were conducted according to the recommendations for research in experimental stroke studies and the current ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). Animals were randomly assigned to the operators by independent persons not involved in data acquisition and analysis. Surgeries and evaluation of all readout parameters were performed while being blinded to the experimental groups.
Determination of infarct size
Assessment of infarct size was performed 24 hours after induction of cerebral ischemia, as described.5 After sacrificing the animal, the brain was removed and sectioned in three consecutive 2-mm thick sections. Sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich) in phosphate-buffered saline and fixated. Pictures were taken and the infarct volume was calculated via planimetry (ImageJ software, National Institutes of Health).
Western blot analysis
Western blot analysis for fibrin/fibrinogen was performed as previously described.5 The 2,3,5-triphenyltetrazolium chloride-stained coronal brain sections of 1 mouse were separated in ipsilateral and contralateral parts, pooled, and homogenized. Immunoreactivity for fibrin(ogen) [anti-fibrin(ogen) pAb 1:500; Acris Antibodies GmbH, Herford, Germany] was detected by western blot analysis and quantified by densitometry using ImageJ software. Actin served as a loading control. The amount of fibrin(ogen) was normalized to the actin signal. The ratio of the normalized ipsilateral fibrin(ogen) band density relative to the normalized band density of contralateral fibrin(ogen) served as a measurement for relative fibrin(ogen) deposition. A detailed description of this assay is provided in the supplemental Methods.
Statistics
Data are expressed as mean ± standard error of the mean. For statistical analysis, the Prism Version 6.0 software (GraphPad Software; La Jolla, CA) was used. Statistical analysis was conducted using one-way analysis of variance followed by the Tukey’s multiple comparison post hoc test to assess variance between FVIII:C levels, cerebral infarct sizes, and fibrinogen deposition in different experimental groups. The Kruskal-Wallis test followed by Dunn’s multiple comparison post hoc test was used to compare plasma VWF antigen levels, tail clip bleeding times, and arterial occlusion times. Values of P < .05 were considered statistically significant.
Results
Generation of chimeric mice expressing VWF only in megakaryocytes or endothelial cells
To determine the contribution of platelet VWF in hemostasis and thrombosis, we generated chimeric mice that produced VWF only in megakaryocytes or ECs, respectively. MNCs were isolated from WT donor mice and transplanted into lethally irradiated VWF KO acceptor mice, resulting in chimeric mice with only platelet VWF (VWF PLT chimeric mice) (Figure 1A). By transplanting VWF KO MNCs into WT acceptor mice, chimeric mice that specifically lack VWF in platelets were obtained (VWF EC chimeric mice) (Figure 1B). No difference in platelet count between chimeric, VWF KO, and WT mice was observed (data not shown). To confirm successful transplantation, the relative amount of VWF-containing platelets was determined via flow cytometry (Figure 1C). As in VWF KO mice, no VWF-positive platelets were detected in VWF EC chimeric mice. The relative amount of VWF-positive platelets in VWF PLT chimeric mice (98.44 ± 1.87%; n = 18) was similar to WT mice (99.94 ± 1.31%; n = 12) (Figure 1C).
Generation and characterization of chimeric mice that lack VWF in either megakaryocytes or endothelial cells. MNCs, isolated from donor WT (black) or VWF KO mice (white), were transplanted into lethally irradiated VWF KO or WT acceptor mice to generate (A) chimeric mice that have VWF only in platelets (VWF PLT) or (B) chimeric mice that specifically lack VWF in their platelets (VWF EC), respectively. (C) Relative amount of VWF-positive platelets detected in WT (n = 17), VWF KO (n = 12), VWF PLT chimeric (n = 18), and VWF EC chimeric (n = 14) mice. (D) VWF antigen (Ag) levels (VWF:Ag) were determined in plasma samples from WT (n = 16), VWF KO (n = 14), VWF PLT chimeric (n = 21), and VWF EC chimeric (n = 8) mice. (E) FVIII activity (FVIII:C) was determined in plasma samples from WT (n = 12), VWF KO (n = 12), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 10) mice. Results are expressed as percentage of WT values. ns, not statistically significant. ***P < .001.
Generation and characterization of chimeric mice that lack VWF in either megakaryocytes or endothelial cells. MNCs, isolated from donor WT (black) or VWF KO mice (white), were transplanted into lethally irradiated VWF KO or WT acceptor mice to generate (A) chimeric mice that have VWF only in platelets (VWF PLT) or (B) chimeric mice that specifically lack VWF in their platelets (VWF EC), respectively. (C) Relative amount of VWF-positive platelets detected in WT (n = 17), VWF KO (n = 12), VWF PLT chimeric (n = 18), and VWF EC chimeric (n = 14) mice. (D) VWF antigen (Ag) levels (VWF:Ag) were determined in plasma samples from WT (n = 16), VWF KO (n = 14), VWF PLT chimeric (n = 21), and VWF EC chimeric (n = 8) mice. (E) FVIII activity (FVIII:C) was determined in plasma samples from WT (n = 12), VWF KO (n = 12), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 10) mice. Results are expressed as percentage of WT values. ns, not statistically significant. ***P < .001.
Absence of VWF-positive platelets in VWF EC chimeric mice confirms previous findings that endocytosis of VWF from plasma to platelet α-granules does not occur.15,19 As expected, platelets do not seem to release significant amounts of VWF into the circulation under normal conditions (Figure 1D). Indeed, normal plasma levels of VWF antigen were found in WT mice (99.9 ± 7.1%; n = 16) and VWF EC chimeric mice (106.3 ± 9.3%; n = 8), whereas no detectable levels of VWF were observed in plasma of VWF KO mice (n = 14) and most VWF PLT chimeric mice (n = 21). However, in some (7 of 21) VWF PLT chimeric mice, there were trace amounts of VWF observed, which could account for the slightly (but nonsignificantly) increased FVIII activity (14.3 ± 1%; n = 16) compared with VWF KO mice (6.3 ± 0.4%; n = 12) (Figure 1E). FVIII activity levels in the VWF EC chimeric mice (83.6 ± 3.1%; n = 10) were slightly decreased when compared with WT mice (100 ± 4.6%; n = 12), but remained in the normal range (Figure 1E). These data further confirm the notion that plasma VWF mainly originates from endothelial cells and that no platelet VWF is constitutively secreted into the circulation under normal circumstances.
Plasma, but not platelet VWF, is the major determinant for normal hemostasis
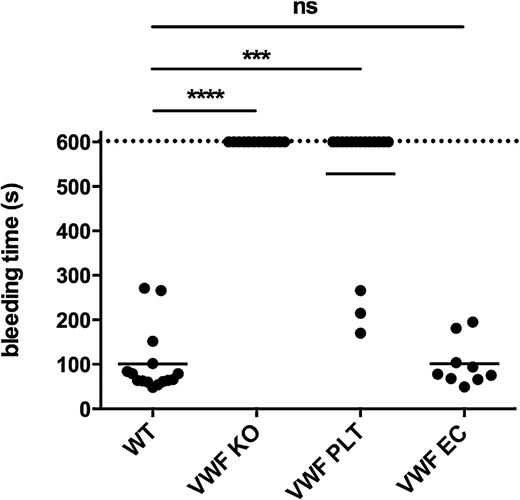
To unravel the specific role of platelet VWF in hemostasis, we used our chimeric mice in a tail clip bleeding assay. After removing 5 mm of the tip of the tail, the time needed to stop bleeding was recorded (Figure 2). As expected, most WT animals stopped bleeding within 3 minutes (100.9 ± 18.7 seconds; n = 15), whereas VWF KO mice were not able to control the bleeding within 10 minutes (>600 seconds; n = 14). Interestingly, most of the VWF PLT chimeric mice also bled longer than 10 minutes (528.2 ± 38.9 seconds; n = 16). Bleeding stopped spontaneously in 3 of 16 mice (19%). In chimeric mice that only produced endothelial cell–derived VWF, bleeding times were comparable to those of WT mice (101.1 ± 17.3 seconds; n = 9). These data suggest that platelet VWF alone is not able to support normal hemostasis and that lack of platelet VWF does not affect bleeding in this model.
Plasma VWF is the major determinant to control bleeding in a tail clip bleeding assay. Five mm of the tail of WT (n = 15), VWF KO (n = 14), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 9) mice was removed. Subsequent tail bleeding in pre-warmed saline was monitored and the time needed to stop the bleeding was recorded. If bleeding did not end within the first 10 minutes, the experiment was ended with a recorded bleeding time of 600 seconds. ns, not statistically significant. ***P < .001; ****P < .0001.
Plasma VWF is the major determinant to control bleeding in a tail clip bleeding assay. Five mm of the tail of WT (n = 15), VWF KO (n = 14), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 9) mice was removed. Subsequent tail bleeding in pre-warmed saline was monitored and the time needed to stop the bleeding was recorded. If bleeding did not end within the first 10 minutes, the experiment was ended with a recorded bleeding time of 600 seconds. ns, not statistically significant. ***P < .001; ****P < .0001.
Plasma, but not platelet VWF, regulates arterial thrombus formation
Ex vivo flow chamber studies have previously shown that platelet-derived VWF plays a role in platelet adhesion to collagen.13-15 However, the exact role of platelet VWF in in vivo thrombus formation in mice has never been studied. To address this question, we performed a FeCl3-induced carotid artery thrombosis model (Figure 3). In WT mice, an occlusive thrombus was consistently formed within 10 minutes (9.2 ± 0.5 minutes; n = 8). In contrast, no occlusive thrombus was formed within the first 50 minutes in VWF KO mice (n = 5). Interestingly, arterial occlusion times in VWF EC chimeric mice (10.6 ± 0.4 minutes; n = 6) were similar to WT mice, whereas no occlusive thrombi were formed in VWF PLT chimeric mice (n = 8). These results indicate that on the one hand, platelet-derived VWF alone is not able to support normal thrombus formation in this model, and that on the other hand, lack of VWF in platelets does not affect normal arterial thrombosis.
Plasma VWF, but not platelet VWF, determines thrombotic occlusion rates in a carotid artery thrombosis model. (A) The carotid artery of WT (n = 8), VWF KO (n = 5), VWF PLT chimeric (n = 8), and VWF EC chimeric (n = 6) mice was exposed and a local injury was generated by topical application of a filter paper saturated with 12% FeCl3. Carotid artery blood flow was monitored and the time needed to form an occlusive thrombus was recorded. If no occlusion occurred within 50 minutes, the experiment was ended (time to occlusion of 50 minutes). (B) Carotid artery (CA) blood flow profiles of WT (black; n = 8), VWF KO (gray; n = 5), VWF PLT chimeric (dashed gray; n = 8), and VWF EC chimeric (dashed black; n = 6) mice were recorded using a laser Doppler flow monitor. ns, not statistically significant.**P < .01; ***P < .001.
Plasma VWF, but not platelet VWF, determines thrombotic occlusion rates in a carotid artery thrombosis model. (A) The carotid artery of WT (n = 8), VWF KO (n = 5), VWF PLT chimeric (n = 8), and VWF EC chimeric (n = 6) mice was exposed and a local injury was generated by topical application of a filter paper saturated with 12% FeCl3. Carotid artery blood flow was monitored and the time needed to form an occlusive thrombus was recorded. If no occlusion occurred within 50 minutes, the experiment was ended (time to occlusion of 50 minutes). (B) Carotid artery (CA) blood flow profiles of WT (black; n = 8), VWF KO (gray; n = 5), VWF PLT chimeric (dashed gray; n = 8), and VWF EC chimeric (dashed black; n = 6) mice were recorded using a laser Doppler flow monitor. ns, not statistically significant.**P < .01; ***P < .001.
Both endothelial cell- and platelet-derived VWF alone are able to mediate ischemic brain injury
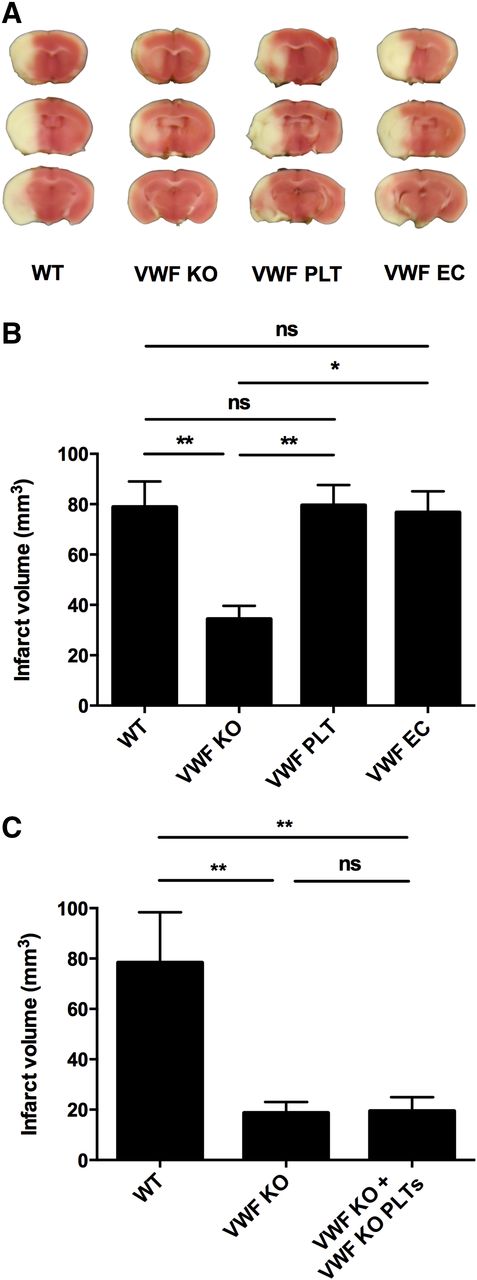
We and others have shown that VWF plays a crucial role in ischemic stroke most probably by recruiting platelets and leukocytes in the reperfused tissue.3-5 Interestingly, absence of ADAMTS13 further aggravates ischemic brain damage, suggesting a specific contribution of UL-VWF multimers in the pathophysiology of stroke.4,12,20 Given the abundance of UL-VWF multimers in α-granules of platelets, we explored the specific contribution of platelet VWF in ischemic brain injury. After 60 minutes of focal cerebral ischemia, blood flow in the middle cerebral artery was restored to allow reperfusion of the ischemic territory. Twenty-four hours after induction of ischemia, cerebral infarction sizes were determined (Figure 4). In accordance with our previous studies,3,5 cerebral infarct sizes observed in VWF KO mice (34.4 ± 5.2 mm3; n = 12) were significantly reduced compared with WT mice (78.9 ± 10.2 mm3; n = 10). As expected, VWF EC chimeric mice developed infarcts that were similar in size to WT animals (76.7 ± 8.4 mm3; n = 9). Surprisingly, however, VWF PLT chimeric mice expressing VWF only in platelets also showed infarct sizes that were similar to WT mice (79.6 ± 8.1 mm3; n = 16). Because cranial irradiation may induce persistent neuroinflammation in C57BL/6J mouse brain21,22 we tested lethally irradiated VWF KO mice transplanted with VWF KO MNCs to exclude any potential effect of irradiation on cerebral injury in our model (Figure 4C). In this separate set of experiments, infarct sizes were still small (18.71 ± 4.31 mm3; n = 7) and comparable with those observed in VWF KO mice (19.43 ± 5.49 mm3; n = 7). Hence, the irradiation procedure had no effect on infarct size in VWF KO animals. Together, these data show that the presence of either platelet VWF or plasma VWF alone is sufficient to cause VWF-mediated ischemic stroke injury.
Platelet-derived VWF alone mediates ischemic stroke injury. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in WT (n = 12), VWF KO (n = 10), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 9) mice. (A) Representative 2,3,5-triphenyltetrazolium chloride staining of 3 consecutive coronal brain sections 24 hours after induction of transient middle cerebral artery occlusion (tMCAO) are shown. White color indicates infarcted area, whereas a pink color shows unaffected brain tissue. (B) Brain infarct volumes as quantified by planimetric analysis 24 hours after tMCAO. (C) To exclude potential irradiation-mediated effects on cerebral injury, transient focal cerebral ischemia was induced in irradiated VWF KO mice transplanted with MNCs derived from VWF KO mice (VWF KO + VWF KO PLTs). Planimetric analysis of brain infarct volumes 24 hours after tMCAO in WT (n = 5), VWF KO (n = 7), and VWF KO + VWF KO PLTs chimeric mice (n = 7). ns, not statistically significant. *P < .05; **P < .01.
Platelet-derived VWF alone mediates ischemic stroke injury. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in WT (n = 12), VWF KO (n = 10), VWF PLT chimeric (n = 16), and VWF EC chimeric (n = 9) mice. (A) Representative 2,3,5-triphenyltetrazolium chloride staining of 3 consecutive coronal brain sections 24 hours after induction of transient middle cerebral artery occlusion (tMCAO) are shown. White color indicates infarcted area, whereas a pink color shows unaffected brain tissue. (B) Brain infarct volumes as quantified by planimetric analysis 24 hours after tMCAO. (C) To exclude potential irradiation-mediated effects on cerebral injury, transient focal cerebral ischemia was induced in irradiated VWF KO mice transplanted with MNCs derived from VWF KO mice (VWF KO + VWF KO PLTs). Planimetric analysis of brain infarct volumes 24 hours after tMCAO in WT (n = 5), VWF KO (n = 7), and VWF KO + VWF KO PLTs chimeric mice (n = 7). ns, not statistically significant. *P < .05; **P < .01.
Platelet VWF-mediated ischemic brain injury is GPIbα-dependent
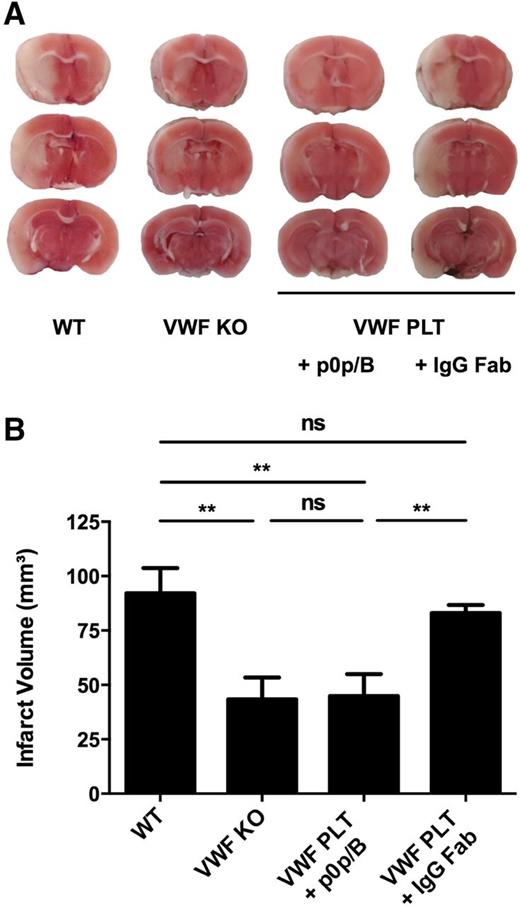
Next we wanted to further elucidate the mechanism by which platelet VWF exacerbates ischemic stroke. The main interactions of VWF in platelet adhesion and thrombus formation are binding to platelet GPIb, collagen, and platelet GPIIb/IIIa. Interestingly, however, Williams et al9 have shown that platelet-derived VWF binds GPIbα with lower affinity than plasma VWF. To test whether binding of platelet VWF to GPIb is important in ischemic stroke, we blocked the VWF-GPIbα axis using an anti-GPIbα (p0p/B) Fab fragment (Figure 5).23 Administration of p0p/B significantly reduced cerebral infarct development in VWF PLT chimeric mice (44.8 ± 10.19 mm3; n = 8) to levels as observed in VWF KO mice (43.38 ± 10.02 mm3; n = 5). These were half the size of those observed in nontreated WT mice (92.19 ± 11.52 mm3; n = 6) or VWF PLT chimeric mice that received IgG control Fab (83.07 ± 3.7 mm3; n = 12). These data suggest that the detrimental effect of platelet VWF in ischemic stroke is mediated by a platelet GPIbα-dependent mechanism.
Blockade of platelet GPIbα reduces platelet VWF-mediated ischemic stroke injury. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in nontreated WT (n = 6) and VWF KO (n = 5) mice, and VWF PLT chimeric mice treated with 100 μg anti-GPIbα Fab (+ p0p/B; n = 8) or rat IgG Fab control (+ IgG Fab; n = 12). (A) Representative 2,3,5-triphenyltetrazolium chloride staining of 3 consecutive coronal brain sections 24 hours after transient middle cerebral artery occlusion (tMCAO). White color indicates infarcted area, whereas a pink color shows unaffected brain tissue. (B) Brain infarct volumes as quantified by planimetric analysis 24 hours after tMCAO. ns, not statistically significant. **P < .01.
Blockade of platelet GPIbα reduces platelet VWF-mediated ischemic stroke injury. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in nontreated WT (n = 6) and VWF KO (n = 5) mice, and VWF PLT chimeric mice treated with 100 μg anti-GPIbα Fab (+ p0p/B; n = 8) or rat IgG Fab control (+ IgG Fab; n = 12). (A) Representative 2,3,5-triphenyltetrazolium chloride staining of 3 consecutive coronal brain sections 24 hours after transient middle cerebral artery occlusion (tMCAO). White color indicates infarcted area, whereas a pink color shows unaffected brain tissue. (B) Brain infarct volumes as quantified by planimetric analysis 24 hours after tMCAO. ns, not statistically significant. **P < .01.
Platelet-derived VWF exacerbates ischemic brain injury by promoting intracerebral thrombosis
To gain further insight on how platelet VWF promotes cerebral injury, we measured fibrin(ogen) deposition in the brain after stroke (Figure 6). In WT mice, the ischemic hemisphere contained almost four times more fibrin(ogen) than the contralateral hemisphere (ratio ipsilateral/contralateral of 3.69 ± 0.37; n = 9) 24 hours after stroke, suggesting thrombotic events occurring during the ischemia/reperfusion phase. In VWF KO mice, significantly less fibrin(ogen) was found in the ipsilateral hemisphere (only a 1.44 ± 0.17-fold increase of contralateral; n = 8). Reconstitution of the platelet VWF compartment in VWF KO mice (VWF PLT chimeric mice) again significantly increased cerebral fibrin(ogen) deposition in the affected hemisphere (ratio ipsilateral/contralateral of 2.99 ± 0.56; n = 7). Interestingly, when VWF PLT chimeric mice were treated with anti-GPIbα Fab, fibrin(ogen) in the ipsilateral hemispheres was reduced (ratio ipsilateral/contralateral of 1.28 ± 0.10; n = 5). Administration of control IgG Fab to VWF PLT chimeric mice did not alter fibrin(ogen) deposition in the brain (ratio ipsilateral/contralateral of 3.75 ± 0.34; n = 6). These data suggest a significant contribution of platelet-derived VWF in intracerebral fibrin(ogen) deposition during ischemic stroke that depends on VWF binding to GPIbα.
Platelet-derived VWF contributes to ischemic brain injury by promoting intracerebral thrombosis. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in WT (n = 9), VWF KO (n = 8), nontreated VWF PLT chimeric (n = 7), anti-GPIbα Fab-treated VWF PLT chimeric (p0p/B, n = 5), and control IgG Fab-treated VWF PLT chimeric (IgG Fab; n = 6) mice. Twenty-four hours after induction of ischemia, the amount of fibrin(ogen) in both the contralateral and ipsilateral hemisphere was determined by semiquantitative western blot analysis. (A) Representative immunoblot showing accumulation of fibrin(ogen) in ipsilateral (I) and contralateral (C) hemispheres. (B) Band intensities from A were quantified by densitometry. Each fibrin(ogen) density signal was first normalized to the corresponding actin density signal. The ratio of the normalized ipsilateral fibrin(ogen) density relative to the normalized contralateral fibrin(ogen) band served as a measurement for relative fibrin(ogen) deposition (ratio ipsilateral/contralateral [ratio IPSI/CONTRA]). ns, not statistically significant. *P < .05; **P < .01; ***P < .001.
Platelet-derived VWF contributes to ischemic brain injury by promoting intracerebral thrombosis. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in WT (n = 9), VWF KO (n = 8), nontreated VWF PLT chimeric (n = 7), anti-GPIbα Fab-treated VWF PLT chimeric (p0p/B, n = 5), and control IgG Fab-treated VWF PLT chimeric (IgG Fab; n = 6) mice. Twenty-four hours after induction of ischemia, the amount of fibrin(ogen) in both the contralateral and ipsilateral hemisphere was determined by semiquantitative western blot analysis. (A) Representative immunoblot showing accumulation of fibrin(ogen) in ipsilateral (I) and contralateral (C) hemispheres. (B) Band intensities from A were quantified by densitometry. Each fibrin(ogen) density signal was first normalized to the corresponding actin density signal. The ratio of the normalized ipsilateral fibrin(ogen) density relative to the normalized contralateral fibrin(ogen) band served as a measurement for relative fibrin(ogen) deposition (ratio ipsilateral/contralateral [ratio IPSI/CONTRA]). ns, not statistically significant. *P < .05; **P < .01; ***P < .001.
Discussion
As a main finding of this study, we showed that platelet VWF is not essential for normal hemostasis and arterial thrombus formation, but this specific pool of VWF can contribute to ischemic brain injury via a GPIbα-dependent mechanism.
Although normal plasma VWF levels were observed in VWF EC chimeric mice, no VWF was detected in their platelets. This observation confirms earlier transplantation studies performed in both mice15 and pigs,19 and this corroborates the absence of VWF in platelets of von Willebrand disease type 3 patients after replacement therapy.24 Hence, our data further support the hypothesis that platelet VWF originates solely from megakaryocytes and is not taken up from plasma, in contrast to fibrinogen, for example. Chimeric mice with VWF only in platelets did not show any significant amounts of VWF in plasma. In addition, no significant increase in circulating FVIII was observed. Similar observations were obtained in chimeric pigs.19 Interestingly, Kanaji et al15 showed trace amounts of circulating VWF in chimeric mice that only have VWF in platelets. One possible explanation of this discrepancy could be a difference in VWF enzyme-linked immunosorbent assay detection sensitivities. Alternatively, slight platelet activation can result in the release of VWF in the plasma.
The contribution of platelet-derived VWF in normal hemostasis is still not totally clear. Case studies in humans suggested that platelet-derived VWF is beneficial for normalization of bleeding.25-28 Our tail clip bleeding experiments show that endothelial cell-derived VWF is the major determinant in the process of normal hemostasis. This concept is further supported by earlier observations of defective hemostasis in mice15 and pigs29,30 with VWF only in platelets. Furthermore, hydrodynamic gene transfer experiments that target the liver for VWF expression also show that VWF present only in plasma can correct the bleeding diathesis of VWF KO mice.17,31 Nevertheless, we observed that a small subset (19%) of mice expressing VWF only in platelets had partially corrected bleeding times, suggesting that in some cases platelet-derived VWF is more or less able to support hemostasis. Similar results were obtained by Kanaji et al15 who showed that 38% of their VWF PLT chimeric mice also had a partially corrected bleeding time. Why bleeding stopped in some of our VWF PLT chimeric mice remains unclear. These mice did not show higher platelet counts nor did they have higher levels of plasma VWF or FVIII. Although less likely, differences in size and quantity of VWF in the platelet granules might contribute to these observations. More studies are needed, preferably using bleeding models that more closely mimic clinically relevant human hemorrhage, to further clarify the specific function of platelet VWF in bleeding.
Platelet VWF is important for initial in vitro platelet adhesion on collagen-coated surfaces.15,30 To test whether this is relevant for in vivo thrombus formation, we used a FeCl3-induced carotid artery thrombosis model. Interestingly, mice having VWF only in platelets could not form occlusive thrombi, whereas chimeric mice with only endothelial cell–derived VWF did so within 10 minutes. Thus, overall arterial thrombus formation does not seem to be largely dependent on VWF in platelets, which is in line with earlier thrombosis studies in chimeric pigs.29 In our model, we were not able to assess initial platelet adhesion rates after carotid artery injury, but lack of VWF in platelets seemed to slow down the rate of blood flow reduction compared with WT mice, an effect that was lost, however, in the total kinetics of occlusive thrombus formation in the large carotid artery. Studies using other thrombosis models would be helpful to further explore the specific role of platelet VWF in thrombus formation.
Increased platelet adhesion at sites of vascular injury could be important in thrombotic pathologies affecting the microcirculation such as ischemic stroke. We and others have shown that VWF KO mice are protected from stroke.3-5 Chimeric mice that lack VWF in platelets developed the same cerebral infarctions as observed in WT animals, suggesting that plasma VWF alone can mediate the VWF-dependent ischemic brain injury. These findings are in agreement with the same large infarctions observed in VWF KO mice reconstituted with plasma VWF via hydrodynamic VWF gene transfer.5 Surprisingly, stroke lesions in VWF PLT chimeric mice were also similar to those seen in WT mice. Indeed, whereas VWF PLT chimeric mice display a phenotype similar to VWF KO animals in the tail clip bleeding and carotid artery thrombosis model, this is not the case in the stroke model. Hence, the contribution of VWF in platelets seems to be specifically relevant in the setting of cerebral ischemia. In addition, we observed an increased intracerebral deposition of fibrin(ogen) in VWF PLT chimeric mice compared with VWF KO mice indicating that local release of platelet VWF can promote intracerebral thrombosis. It is tempting to speculate that platelets adhere to activated endothelium (or subendothelium) in the area of ischemia/reperfusion and upon activation, secrete their granular contents, including large amounts of UL-VWF. This may result in the formation of platelet-derived VWF strings that may even be more resistant to ADAMTS13 proteolysis.10,32 Similar to endothelial cell–derived VWF strings, VWF strings from platelets could recruit new platelets and inflammatory cells resulting in platelet/leukocyte plugs that block the microvasculature and contribute to the no-reflow phenomenon. Interestingly, such platelet derived-VWF strings have been shown in vitro.33 Future studies specifically addressing the inflammatory nature of platelet-derived VWF will definitely shed more light on its role in this thrombo-inflammatory process.
Despite in vitro data showing that purified platelet VWF has a lower affinity for GPIbα than plasma VWF,9 we found that platelet VWF mediates ischemic brain injury via a GPIb-dependent mechanism. This finding further supports the crucial importance of the VWF-GPIb axis in stroke.5-7,23,34 Although p0p/B is not cytotoxic to platelets,23 we observed a small decrease in platelet count in p0p/B-treated mice compared with the control group (657.4 ± 115.6 vs 895.7 ± 107.1, respectively; P < .01). This difference in platelet count cannot, however, account for the observed difference in infarct size as 10% of normal platelet counts is already sufficient to induce full brain infarction after transient ischemic stroke.35
In conclusion, our data shed an unexpected new light on the activity of platelet VWF and expand our insights on VWF-mediated mechanisms underlying ischemic stroke. This study further supports the idea that blocking the GPIb-VWF axis would be an interesting novel treatment strategy in stroke.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Verhenne, N. Hertsens, and J.-P. Coutant (Department of Radiotherapy, AZ Groeninge Campus Loofstraat, Kortrijk, Belgium) for their help with the irradiation of mice.
This work was supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO G.0298.12) and the Deutsche Forchsungsgemeinschaft (SFB 688).
Authorship
Contribution: S.V. and S.F.D.M. designed research and wrote the manuscript; S.V., F.D., S.L., A.V., I.P., and S.F.D.M. performed experiments; S.V., F.D., S.L., and S.F.D.M analyzed data and interpreted results; A.L., C.K., and B.N. provided essential materials and equipment; and F.D., C.K., B.N., H.D., and K.V. helped editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon F. De Meyer, Laboratory for Thrombosis Research, KU Leuven, Campus Kulak Kortrijk, E. Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: simon.demeyer@kuleuven-kulak.be.



![Figure 6. Platelet-derived VWF contributes to ischemic brain injury by promoting intracerebral thrombosis. Transient focal cerebral ischemia was induced by 60 minutes occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion in WT (n = 9), VWF KO (n = 8), nontreated VWF PLT chimeric (n = 7), anti-GPIbα Fab-treated VWF PLT chimeric (p0p/B, n = 5), and control IgG Fab-treated VWF PLT chimeric (IgG Fab; n = 6) mice. Twenty-four hours after induction of ischemia, the amount of fibrin(ogen) in both the contralateral and ipsilateral hemisphere was determined by semiquantitative western blot analysis. (A) Representative immunoblot showing accumulation of fibrin(ogen) in ipsilateral (I) and contralateral (C) hemispheres. (B) Band intensities from A were quantified by densitometry. Each fibrin(ogen) density signal was first normalized to the corresponding actin density signal. The ratio of the normalized ipsilateral fibrin(ogen) density relative to the normalized contralateral fibrin(ogen) band served as a measurement for relative fibrin(ogen) deposition (ratio ipsilateral/contralateral [ratio IPSI/CONTRA]). ns, not statistically significant. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/14/10.1182_blood-2015-03-632901/4/m_1715f6.jpeg?Expires=1769082712&Signature=R0acedTor5c-OxnS-WfYQqI1uWCXUfNfcw8C4jT2w15bIfXw9umD0mgPuINFmbap7UoGZX4Ig1OWPDH5viiZRoawTQQhzjNWMCLdwDHo2s02gvMLehA9Kj91YZnRFtJ7S~S2pyJGBt79SPuZdBU3LcJhry6Hr2s6SCcUp39OTN2CxCXJe3FUq5XmUzLXHPfrc750v-f9MsZOALFoed4beYrb1IR06cV-ZdI-p0jz4PVYR4vGMClgLIFnDl9vpR66ZopPcPBaCPuv51tFPj-AtTJnrXHi3gR29LU6o3svZJPz02oPvjvKYOKlJ-~GVrJtRjOk7zEulwif7-AnrrGl1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)