Key Points
Urinary 3-IS levels predict outcome after ASCT and are associated with antibiotics and NOD2/CARD15 variants.
Abstract
Indole, which is produced from l-tryptophan by commensal bacteria expressing tryptophanase, not only is an important intercellular signal in microbial communities, but also modulates mucosal barrier function and expression of pro- and anti-inflammatory genes by intestinal epithelial cells. Here, we hypothesized that decreased urinary excretion of 3-indoxyl sulfate (3-IS), the major conjugate of indole found in humans, may be a marker of gut microbiota disruption and increased risk of developing gastrointestinal (GI) graft-versus-host-disease. Using liquid chromatography/tandem mass spectrometry, 3-IS was determined in urine specimens collected weekly within the first 28 days after allogeneic stem cell transplantation (ASCT) in 131 patients. Low 3-IS levels within the first 10 days after ASCT were associated with significantly higher transplant-related mortality (P = .017) and worse overall survival (P = .05) 1 year after ASCT. Least absolute shrinkage and selection operator regression models trained on log-normalized counts of 763 operational taxonomic units derived from next-generation sequencing of the hypervariable V3 region of the 16S ribosomal RNA gene showed members of the families of Lachnospiraceae and Ruminococcaceae of the class of Clostridia to be associated with high urinary 3-IS levels, whereas members of the class of Bacilli were associated with low 3-IS levels. Risk factors of early suppression of 3-IS levels were the type of GI decontamination (P = .01), early onset of antibiotic treatment (P = .001), and recipient NOD2/CARD15 genotype (P = .04). In conclusion, our findings underscore the relevance of microbiota-derived indole and metabolites thereof in mucosal integrity and protection from inflammation.
Introduction
Allogeneic stem cell transplantation (ASCT) constitutes a potential curative therapy for various hematologic malignancies, bone marrow failure, and immune deficiency syndromes. However, this treatment is still associated with a high risk of mortality because of infectious complications and acute graft-versus-host disease (GVHD). A significant part of these severe complications originates from the gastrointestinal (GI) tract.1
The introduction of 16S ribosomal RNA (rRNA) sequencing has provided novel insights into the diversity and complexity of the gut ecosystem.2 Loss of a diverse composition of the microbiome has been associated with a variety of diseases including inflammatory bowel and autoimmune diseases. At least in part this may be because of the increasingly recognized role that commensal bacteria play in maintaining immunologic homeostasis and epithelial integrity and in exerting anti-inflammatory effects and intestinal tolerance by inducing regulatory T cells.3,4
Recent studies have demonstrated an association between intestinal bacterial diversity in both mouse models and humans and outcome of ASCT.5-7 A significantly higher transplant -related mortality (TRM) was observed in patients with low intestinal microbiome diversity, whereas patients with a microbiome dominated by commensal bacteria showed a better overall survival (OS) within 3 years after transplantation.8
Recently, we observed urinary levels of 3-indoxyl sulfate (3-IS), which originates from the degradation of tryptophan to indole by intestinal microbiota followed by microsomal oxidation to indoxyl and sulfonation in the liver, to be associated with gut microbiota disruption in patients undergoing ASCT.6 We investigated whether urinary 3-IS levels at the time of ASCT and early thereafter can serve as predictors of outcome as measured by TRM and OS. Our findings not only reveal a prognostic impact of urinary 3-IS levels, but also indicate a direct association of 3-IS levels with the presence of members of the Firmicute families Lachnospiraceae and Ruminococcaceae in the gut microbiota.
Patients, materials, and methods
Patients
A total of 131 adult patients undergoing ASCT between September 2008 and February 2014 at the University Medical Center of Regensburg were enrolled in this prospective single-center study. We expanded our previous pilot cohort of 51 patients with an additional cohort of 80 consecutive patients undergoing ASCT between April 2012 and February 2014.9 The study had been approved by the Ethics Committee of the University Medical Center of Regensburg. After written informed consent, stool and urinary specimens were collected at a minimum of 6 different time points starting prior to admission until day 28 after ASCT: prior to admission, day of transplantation (day −2 to +2), at least once between days +2 and +10, and approximately day +14 (days +11 to +17), day +21 (days +18 to +24), and day +28 (days +25 to +30), respectively. All specimens were stored at −80°C until analysis.
Mean age of the patients was 50.8 (± 12.1) years. Underlying diseases were acute leukemia (n = 72), lymphatic neoplasia (n = 25), myelodysplastic syndrome (n = 17), chronic myelogenous leukemia (n = 14), and aplastic anemia (n = 3). Thirty-seven patients received grafts from HLA-identical siblings, and 94 patients received grafts from matched unrelated donors. Nineteen and 112 patients, respectively, were treated with full and reduced intensity conditioning. All patients received prophylactic antibiotics from start of conditioning until engraftment. Originally, patients (n = 57) received ciprofloxacin and metronidazole. Starting in April 2012, patients (n = 74) were treated instead with rifaximin, a topical rifamycin derivative with low GI resorption, to prevent the emergence of vancomycin-resistant enterococci. In case of neutropenic infectious complications, piperacillin/tazobactam was used for empiric first-line therapy followed by meropenem and vancomycin as second-line therapy. Overall, 117 out of 131 patients (89.3%) required antibiotic treatment during neutropenia.
Analysis of urinary 3-IS
Analysis of 3-IS was performed by reversed-phase liquid chromatography–electrospray ionization–tandem mass spectrometry in negative ion multiple reaction monitoring mode using method parameters recently described.10 In contrast to the original method, quantification was performed with 3-IS-d4 (Toronto Research Chemicals, Toronto, ON, Canada) instead of isatin as internal standard. The transition m/z 216.0 (Q1) to m/z 80.0 (Q3) was monitored for the deuterated 3-IS. For sample preparation, 10 μL of urine was mixed with 10 μL of internal standard (10 µM 3-IS-d4 in water) and diluted with water (PURELAB Plus water) to a final volume of 100 μL. Gradient elution with mobile phase A (0.1% formic acid in water, volume to volume ratio) and mobile phase B (0.1% formic acid in acetonitrile, volume to volume ratio) began with a 1-minute linear increase from 0% to 50% B, hold at 50% B for 4 minutes, 5 to 6 minutes from 50% to 100% B, hold at 100% B for 1 minute, return to 0% B at 7.1 minutes, and equilibration for 3.9 minutes.11 The lower limit of quantitation (LLOQ) was 4 nmol/L. Values below the LLOQ were substituted with LLOQ/2 for subsequent statistical analyses. The upper limit of quantitation was 30 µmol/L. All specimens that had yielded initially concentrations above the upper limit of quantitation were diluted and reanalyzed. Finally, obtained concentration values were corrected to urinary creatinine (crea) as described previously.6
Genotyping of NOD2/CARD15
To assess a potential association between NOD2/CARD15 polymorphism and urinary 3-IS levels, genomic DNA from the 131 study participants and their corresponding donors was prepared from peripheral blood cells (EDTA blood) prior to admission for ASCT. TaqMan polymerase chain reaction (PCR) for single-nucleotide polymorphisms 8, 12, and 13 for the NOD2/CARD15 gene was performed as previously described.11 Because the study group included only a single case each of compound heterozygous or homozygous mutation, the NOD2/CARD15 “risk allele” was defined as any mutation in single-nucleotide polymorphisms 8, 12, or 13.
Bioinformatics and data analysis
To assess microbial species associated with 3-IS levels, we reanalyzed the hypervariable V3 region 16S rRNA gene sequences that had been previously generated on a Roche Diagnostics 454 GS FLX sequencer employing GS FLX titanium chemistry,6 using the QIIME pipeline.12 Reads were quality filtered and trimmed with default settings. The remaining sequences were combined into operational taxonomic units (OTUs) by using the Quantitative Insights Into Microbial Ecology (QIIME) script pick_closed_reference_otus.py against the SILVA ribosomal RNA gene database (release 111, available at http://www.arb-silva.de/download/archive/qiime/) at 97% sequence identity.13 Taxonomy was assigned as provided by SILVA DB.
Based on the resulting read count data, the Simpson index of diversity was calculated for quantification of bacterial intestinal diversity and correlated with matching urinary levels of 3-IS using the Pearson correlation coefficient. The microbiome signature to predict urinary 3-IS levels was computed using least absolute shrinkage and selection operator (LASSO) regression models trained on the profile of log-normalized counts of 763 OTUs with >60 counts across the 70 stool specimens, for which also urinary 3-IS levels were available, as predictors. All predictor variables were scaled to standard units to allow for a fair comparison of regression coefficients across predictors. Cross-validated predictions were compared with observed urinary log (3-IS/mmol crea) levels. The sparseness of the model was calibrated for every cross-validation run separately in a nested cross-validation loop. Finally, a single model was trained on the full data set, and predictors with nonzero regression coefficients were reported as signature OTUs. Computations were done in R using the glmnet package.14
Normally and nonnormally distributed continuous data are presented as mean (standard deviation) or median (range), respectively. Accordingly, either a 2-sided t test or the Mann-Whitney U test was used to perform group comparisons. Absolute and relative frequencies were given for categorical data and compared between study groups by χ2 tests. Multivariate logistic regression including gut decontamination, use of systemic antibiotics, and NOD2/CARD15 mutation was performed to search for factors influencing urinary 3-IS concentrations. Kaplan-Meier analysis was performed to assess survival and nonrelapse mortality, and Cox regression was used for multivariate assessment of risk factors. IBM SPSS Statistics 22 (SPSS Inc., Chicago, IL) was used for these analyses.
Results
Low urinary 3-IS levels are associated with poor outcome after ASCT
Patients with TRM within the first 12 months after ASCT (N = 22) showed lower urinary 3-IS levels within the first 10 days after ASCT with a median of 1.3 (0.0-29.6) µmol/mmol crea as compared with all other patients (N = 109) with 8.4 (0.0-101.9) µmol/mmol crea (P = .010). In contrast, 3-IS levels prior to ASCT and at later time points did not differ significantly between patients with and without TRM.
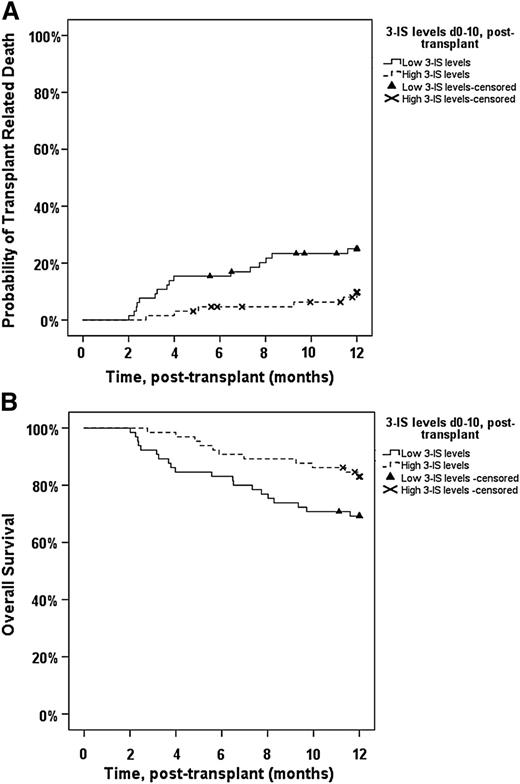
Patients were next classified into either low or high 3-IS based on 3-IS concentrations between day 0 and day 10 after ASCT. In case of several available 3-IS measurements within this period, we chose the mean of all 3-IS values obtained between day 0 and 10 after ASCT. Kaplan-Meier estimates for TRM and OS for each group are shown in Figure 1. Patients with low urinary 3-IS levels (≤6.9 µmol/mmol crea) displayed a significantly higher TRM in the first 12 months after ASCT than patients with high (>6.9 µmol/mmol crea) urinary 3-IS concentrations (P = .017; Figure 1A). GI GVHD-related complications were the primary cause of TRM in 19 of 22 (86.4%) patients (P < .001). The remaining 3 patients died of Epstein-Barr virus–associated posttransplant lymphoproliferative disorder, fungal septicemia, and hepatorenal syndrome, respectively. Similarly, OS within the first year after ASCT was reduced in the low-3-IS group (P = .05; Figure 1B). Relapse-related mortality did not differ between the low- and high-3-IS groups (not significant). The negative effect of low 3-IS levels on TRM persisted until the most recent follow-up in January 2015 (P = .02; supplemental Figure 1; available on the Blood Web site), and the effect on OS was not any longer significant (P = .07; supplemental Figure 2).
One-year TRM in relation to median 3-IS level within the first 10 days after ASCT. (A) One-year TRM in patients with low 3-IS levels (≤6.9 µmol/mmol crea, n = 75) between days 0 and 10 after ASCT was significantly increased (P = .017) as compared with patients with high 3-IS levels (n = 55). (B) One-year OS in patients with low levels (≤6.9 µmol/mmol crea, n = 75) of 3-IS between days 0 and 10 after ASCT was significantly decreased (P = .05) as compared with patients with high 3-IS levels (n = 55).
One-year TRM in relation to median 3-IS level within the first 10 days after ASCT. (A) One-year TRM in patients with low 3-IS levels (≤6.9 µmol/mmol crea, n = 75) between days 0 and 10 after ASCT was significantly increased (P = .017) as compared with patients with high 3-IS levels (n = 55). (B) One-year OS in patients with low levels (≤6.9 µmol/mmol crea, n = 75) of 3-IS between days 0 and 10 after ASCT was significantly decreased (P = .05) as compared with patients with high 3-IS levels (n = 55).
Table 1 shows the results of the Cox proportional hazards analysis for TRM. Low urinary 3-IS levels between day 0 and 10 after ASCT were significantly associated with increased risk of TRM (P = .013), whereas classical risk factors such as age, stage, donor type, conditioning, median duration of systemic antibiotic therapy, and duration of neutropenia were not. The same held for NOD2/CARD15 polymorphism (data not shown).
Multivariate risk factor analysis for TRM in the first 12 mo after ASCT
| Risk factor . | P . | HR . | 95% CI for HR . |
|---|---|---|---|
| Low 3-IS level days 0 to 10 (n = 75) | .013 | 3.895 | 1.329-11.412 |
| Patient’s age (>50 y, n = 81) | .078 | 2.499 | 0.903-6.916 |
| Donor (MUD, n = 94) | .861 | 1.096 | 0.394-3.046 |
| Stage of underlying disease (advanced, n = 45) | .623 | 1.250 | 0.514-3.040 |
| Conditioning (RIC, n = 112) | .943 | 0.973 | 0.464-2.041 |
| Length of neutropenia (median 21 d, range 0-40 d) | .636 | 1.316 | 0.422-4.103 |
| Duration of antibiotic therapy (median 19 d, range 0-40 d) | .351 | 0.627 | 0.234-1.674 |
| Risk factor . | P . | HR . | 95% CI for HR . |
|---|---|---|---|
| Low 3-IS level days 0 to 10 (n = 75) | .013 | 3.895 | 1.329-11.412 |
| Patient’s age (>50 y, n = 81) | .078 | 2.499 | 0.903-6.916 |
| Donor (MUD, n = 94) | .861 | 1.096 | 0.394-3.046 |
| Stage of underlying disease (advanced, n = 45) | .623 | 1.250 | 0.514-3.040 |
| Conditioning (RIC, n = 112) | .943 | 0.973 | 0.464-2.041 |
| Length of neutropenia (median 21 d, range 0-40 d) | .636 | 1.316 | 0.422-4.103 |
| Duration of antibiotic therapy (median 19 d, range 0-40 d) | .351 | 0.627 | 0.234-1.674 |
Low urinary 3-IS levels between days 0 and 10 after ASCT were significantly associated with increased risk of TRM (n = 130). In the table, numbers for high-risk groups are indicated for categorical variables. Significance level <.05.
CI, confidence interval; HR, hazard ratio; MUD, matched unrelated donor; RIC, reduced intensity conditioning.
3-IS levels can be predicted from the gut microbiota composition
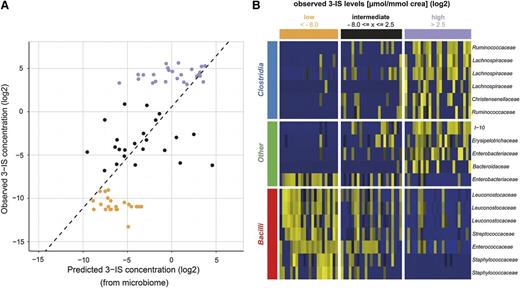
We next asked whether it is possible to predict urinary log 3-IS concentrations if one knows the microbiome composition of a matching stool specimen. To fully exploit the profiles, we used LASSO regression models trained on the profile of log-normalized counts of the 763 OTUs with >60 counts across stool specimens as predictors for log(3-IS/mmol crea) levels and compared cross-validated predictions with observed urinary log(3-IS/mmol crea) levels in Figure 2A. The predicted and observed log(3-IS) levels show a Pearson correlation coefficient of r = 0.703 (P < .001). These associations suggests that knowing the intestinal microbiome composition may allow prediction of urinary 3-IS levels. The OTUs with nonzero regression coefficients of a model trained on 70 stool specimens are summarized in supplemental Table 1 (the respective FASTA files can be found in supplemental Table 2). The LASSO model combines standardized counts of a total of 18 OTUs to a weighted average that is predictive of 3-IS concentrations. Compared with the correlation between the Simpson index of diversity and urinary 3-IS levels (r = 0.252, P = .035), this microbiome signature index correlates more strongly with observed 3-IS concentrations. Hence, not only the diversity of the microbiome but its specific composition is indicative of urinary 3-IS. Interestingly, the majority of OTUs associated with high urinary 3-IS levels belong to the families of Lachnospiraceae, a prominent member of which is Eubacterium rectale, and Ruminococcaceae, both of which belong to the class of Clostridia (Figure 2B). In contrast, low urinary 3-IS levels were primarily associated with members of the class of Bacilli, in particular members of the order of Lactobacillales. It is important to note that inclusion in the model does not necessarily mean that these OTUs are either capable or incapable of converting tryptophan to indole. The model may also include OTUs that do not produce indole themselves but grow either better or worse in the presence of indole.
Microbiome composition is associated with urinary 3-IS levels. (A) LASSO regression was used to predict 3-IS levels from microbiome compositions in cross-validation. Shown is a scatter plot of predicted vs observed urinary 3-IS/mmol crea concentrations. Both axes are plotted on a logarithmic scale. The Pearson correlation coefficient r is 0.703 (P < .001). (B) Heat map representation of the relative abundance of the 18 predictive OTUs in n = 70 stool specimens. The abundance increases from blue to yellow.
Microbiome composition is associated with urinary 3-IS levels. (A) LASSO regression was used to predict 3-IS levels from microbiome compositions in cross-validation. Shown is a scatter plot of predicted vs observed urinary 3-IS/mmol crea concentrations. Both axes are plotted on a logarithmic scale. The Pearson correlation coefficient r is 0.703 (P < .001). (B) Heat map representation of the relative abundance of the 18 predictive OTUs in n = 70 stool specimens. The abundance increases from blue to yellow.
Factors associated with low urinary levels of 3-IS: antibacterial prophylaxis, early treatment with broad-spectrum antibiotics, and NOD2/CARD15 genotype
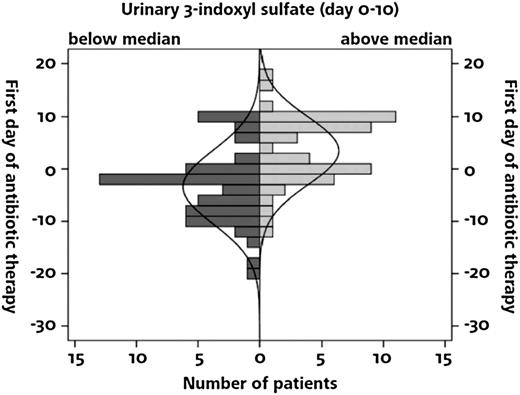
Searching for factors that may be associated with 3-IS, we found significantly lower 3-IS concentrations in patients receiving ciprofloxacin/metronidazole (N = 56) compared with patients receiving rifaximin (N = 74) for gut decontamination (Table 2). A similar effect was observed for the prolonged or early use of systemic antibiotics. In patients with low urinary 3-IS levels, systemic antibiotic treatment had been started more frequently prior to ASCT as compared with patients with high 3-IS levels (Figure 3). There was no difference in 3-IS levels with respect to intensity of conditioning, stage of underlying disease, or patient’s age. In addition, NOD2/CARD15 risk alleles affected 3-IS levels (Table 2). In multivariate data analysis, type of gut decontamination, early treatment of broad-spectrum antibiotics prior to ASCT, and presence of a NOD2/CARD15 risk allele in ASCT recipients were independent risk factors for low levels of 3-IS within the first 10 days after ASCT (Table 3). The mean urinary 3-IS level in ASCT recipients carrying any of the 3 NOD2/CARD15 risk alleles tested were 2.8 µmol/mmol crea (range 0-9.7). If both donor and recipient carried a risk allele, the mean was 3.2 µmol/mmol crea (range 0-12.4). In contrast, if only the donor carried a risk allele, the mean urinary 3-IS level was 12.5 µmol/mmol crea (range 0-63.6), which was similar to the mean level of 12.7 µmol/mmol crea (range 0-101.9) observed when both donor and recipient carried the wild-type allele only. After day 10, the difference in mean urinary 3-IS concentration disappeared between ASCT recipients carrying a risk allele (0.1 µmol/mmol crea; range 0.0-29.9) and those homozygous for the wild-type allele (0.1 µmol/mmol crea; range 0.0-59.5).
Analysis of factors influencing urinary 3-IS concentrations
| . | 3-IS median (range) . | P . |
|---|---|---|
| Use of antibiotics | ||
| Gut decontamination: rifaximin vs Cipro/Metro (74/56) | 12.9 (0.0-101.9) vs 5.3 (0.0-40.2) | .008 |
| Systemic antibiotics in case of neutropenic infections before ASCT (55/71): without or with | 13.7 (0.0-63.6) vs 0.3 (0.0-101.9) | <.0001 |
| Conditioning: RIC vs standard (112/19) | 8.4 (0.0-101.9) vs 2.4 (0.0-29.6) | NS |
| NOD2/CARD15 genotype: wild type (104)/risk allele (26) | 9.2 (0.0-101.9) vs 0.6 (0.0-20.9) | .002 |
| Stage of underlying disease: early or intermediate (85)/late (45) | 6.6 (0-63.6) vs 6.9 (0-101.9) | NS |
| Patient age ≤50 y (49)/>50 y (81) | 4.5 (0-59.4) vs 11.9 (0-101.9) | NS |
| . | 3-IS median (range) . | P . |
|---|---|---|
| Use of antibiotics | ||
| Gut decontamination: rifaximin vs Cipro/Metro (74/56) | 12.9 (0.0-101.9) vs 5.3 (0.0-40.2) | .008 |
| Systemic antibiotics in case of neutropenic infections before ASCT (55/71): without or with | 13.7 (0.0-63.6) vs 0.3 (0.0-101.9) | <.0001 |
| Conditioning: RIC vs standard (112/19) | 8.4 (0.0-101.9) vs 2.4 (0.0-29.6) | NS |
| NOD2/CARD15 genotype: wild type (104)/risk allele (26) | 9.2 (0.0-101.9) vs 0.6 (0.0-20.9) | .002 |
| Stage of underlying disease: early or intermediate (85)/late (45) | 6.6 (0-63.6) vs 6.9 (0-101.9) | NS |
| Patient age ≤50 y (49)/>50 y (81) | 4.5 (0-59.4) vs 11.9 (0-101.9) | NS |
Significant differences in urinary 3-IS levels were observed depending on the antibiotics administered for gut decontamination, the use of systemic antibiotics prior to ASCT, and NOD2/CARD15 genotype, respectively. Parentheses indicate number of valid cases; in 4 cases, no data for systemic antibiotic therapy prior to ASCT were available. Significance level <.05.
Cipro/Metro, ciprofloxacin and metronidazole; NS, not significant; RIC, reduced intensity conditioning.
Suppression of 3-IS in relation to start of antibiotic treatment in 117 patients requiring antibiotic treatment. Systemic antibiotic therapy before ASCT was more frequently observed in patients with 3-IS levels below the median.
Suppression of 3-IS in relation to start of antibiotic treatment in 117 patients requiring antibiotic treatment. Systemic antibiotic therapy before ASCT was more frequently observed in patients with 3-IS levels below the median.
Multivariate analysis of risk factors for urinary 3-IS levels between days 0 and 10 after ASCT
| . | Multivariate analysis (HR, 95% CI) . | P . |
|---|---|---|
| Gut decontamination: rifaximin vs Cipro/Metro | 0.3 (0.1-0.7) | .01 |
| Systemic antibiotics before ASCT: without or with | 0.1 (0.1-0.3) | <.0001 |
| NOD2/CARD15 genotype: wild type/risk allele | 0.3 (0.1-1.0) | .04 |
| . | Multivariate analysis (HR, 95% CI) . | P . |
|---|---|---|
| Gut decontamination: rifaximin vs Cipro/Metro | 0.3 (0.1-0.7) | .01 |
| Systemic antibiotics before ASCT: without or with | 0.1 (0.1-0.3) | <.0001 |
| NOD2/CARD15 genotype: wild type/risk allele | 0.3 (0.1-1.0) | .04 |
Type of gut decontamination, use of systemic antibiotics prior to ASCT, and NOD2/CARD15 polymorphism are independent parameters for low 3-IS levels (n = 126). Significance level <.05.
Discussion
In this prospective study, we report that low urinary 3-IS levels between days 0 and 10 after ASCT are associated with poor early and long-term outcome because of an increased frequency of lethal complications mostly as a consequence of GI GVHD. Further, a LASSO regression model trained on the profile of log-normalized counts of 763 OTUs that were derived from next-generation sequencing of the hypervariable V3 region of the 16S rRNA gene identified an 18-OTU signature that predicts 3-IS levels in cross-validation. Among the signature OTUs, members of the Lachnospiraceae and Ruminococcaceae, which belong to the order of Clostridiales, are found in cases with high urinary 3-IS levels, whereas members of the orders of Lactobacillales and Bacillales were found in cases with low levels. Interestingly, among the OTUs associated with low urinary 3-IS levels was AY033814 (supplemental Table 1), which represents a cluster of hypervariable V3 16S rRNA sequence reads with 97% similarity, including among others the enterococcal strains Enterococcus faecium and Enterococcus gilvus. This is in line with our previous study, in which we observed E. faecium significantly more frequently posttransplant, especially in patients with subsequent or active GI GVHD.6
These observations suggest mechanisms for how microbiota might contribute to protection from intestinal inflammation and possibly GVHD. First, indole and its metabolites may be directly involved. So far, 3-IS has been known as a mediator of endothelial damage and cardiovascular disease in patients suffering from renal failure.15 However, our results appear to support laboratory findings that microbiota-derived indole and derivatives thereof, such as indole-3-aldehyde and 5-hydroxyindole, do not only support survival of mixed microbial communities16,17 but also exert bacteriostatic effects on gram-negative enteric bacilli and certain cocci,18,19 provide colonization resistance to Candida albicans,17 and strengthen epithelial cell barrier properties and protection from inflammation by simultaneously decreasing and increasing expression of proinflammatory and anti-inflammatory cytokines, respectively.17,20 In addition, 3-IS was described to directly regulate T-cell differentiation by stimulating Th2 responses, which may also contribute to GVHD protection.21
Second, the association of Lachnospiraceae with high 3-IS-levels is of major interest. This does not imply that the identified OTUs all produce and release indole; they may just grow better in the presence of indole and some of its derivatives, which have proved capable of slowing the growth of other bacteria such as gram-negative enteric bacilli.18,19 Conversely, the latter will grow better in the absence of indole. Independent of the biological effects of indole within microbial communities and on the human host, the data support the recent finding that Lachnospiraceae, and in particular members of the genus Blautia, which is represented in the present data set by OTU AY990000 (supplemental Table 1), protect against GI GVHD.8,22 Closely related is the genus Roseburia, which has been associated with the generation of the short-chain fatty acid butyrate,23 which is an inducer of intestinal regulatory T cells.3,24 Thus, early loss of bacteria of these genera may result in massive disruption of intestinal immunoregulation25 and contribute to development of GVHD and associated complications. Interestingly, it is hypothesized that disruption of the microbiome by the use of antibiotics in the first months after birth may be responsible for the development of autoimmune disorders in children as microbiota shifts occur in a sensitive period of immune reconstitution.26-28 As the early period after ASCT also represents a period of immune reconstitution, this vulnerability might explain why microbiota changes immediately after ASCT trigger long-term complications. Further studies are needed to address this potential link.
Not unexpectedly, urinary 3-IS levels early after transplantation were influenced by the use of antibiotics for prophylaxis and treatment of neutropenic infections. This effect was more pronounced when broad-spectrum antibiotics were started prior to ASCT. Metronidazole, which has been used for gut decontamination, and both piperacillin/tazobactam and meropenem, which are the preferred antibiotics used for treatment of neutropenic infection, suppress Clostridiales and, thus, may contribute to low urinary 3-IS levels.
Finally, we have found a highly interesting and as yet not described association between urinary 3-IS levels and NOD2/CARD15 variants, which may explain partially our previously described association of NOD2/CARD15 variants with poor outcome following ASCT and discussed effects of decontamination.29 NOD2/CARD15 has been shown to regulate production of antimicrobial peptides both in Paneth cells30 and in neutrophils. Therefore, NOD2/CARD15 deficiency might trigger microbiota shifts resulting in increased inflammatory activities as already demonstrated in inflammatory bowel disease.31 Most of these shifts again occur among Clostridiales. Further, we and others have reported increased bacterial translocation in inflammatory bowel disease patients with NOD2/CARD15 deficiency.32,33 Thus, the effect of NOD2/CARD15 deficiency on 3-IS levels might be more indirect by favoring bacterial translocation resulting in fever and early systemic antibiotic treatment. The use of systemic antibiotics then in turn aggravates the risk of intestinal microbial disruption. More detailed and sequential 16S rRNA sequencing studies as well as experimental approaches are needed to describe the exact interaction of NOD2/CARD15 and urinary 3-IS levels.
Our study has several implications for clinical management of patients, if the results are confirmed in independent prospective validation trials. First, monitoring of urinary levels of 3-IS and other bacterial metabolites may be a more feasible approach to monitor microbiome changes in the clinical setting. Second, loss of Clostridiales associated with production of indole or dependent on 3-IS for growth may be directly involved in regulation of intestinal inflammation in the setting of ASCT. This is also suggested by the recent observation of Shono et al34 in mice, in which antibiotics destroying Clostridiales increased GVHD-related pathology and mortality, whereas introduction of Clostridiales improved survival.8 Thus, careful selection of strategies of decontamination and antibiotic treatment in combination with pre-, pro-, and postbiotics may contribute to improved microbiota diversity and possibly affect outcome. Finally, intestinal immune reconstitution might have a much broader impact on development of at least peripheral tolerance after ASCT and, therefore, should be in the focus of future research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Heike Bremm and Constanze Winter for collecting and cryopreserving patient specimens, as well as Nadine Nuernberger for performing 3-IS analyses.
This work was supported by the German Research foundation (DFG, KFO “Elite”). This work was partially supported by grants from the Regensburg Center for Interventional Immunology and the Marie Curie Initial Training Network Celleurope, European Commission. The project on which this publication is based was partially supported by grants from the University of Regensburg, Medical Center (ReForM).
Authorship
Contribution: D.W., E.H., P.J.O., and W.H. were involved in conception and design of the study; D.W. and J.H. were responsible for collection of specimens; K.D. performed measurements of 3-IS levels; A.H., J.K., and A.G. performed bacterial analysis; R.S., F.S., and M.W. contributed to statistical data analysis; D.W. and E.H. collected and analyzed clinical data and, together with P.J.O., wrote the manuscript; and all authors read and corrected the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ernst Holler, Department of Hematology and Oncology, Internal Medicine III, University Medical Center Regensburg, Franz-Josef-Strauß-Allee 11, 93053 Regensburg, Germany; e-mail: ernst.holler@ukr.de.
References
Author notes
K.D. and E.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal