Key Points
GILZ-deficient mice develop B-cell lymphocytosis.
GILZ deficiency precludes GC-mediated B-cell apoptosis.
Abstract
Glucocorticoids (GC) are widely used as antiinflammatory/immunosuppressive drugs and antitumor agents in several types of lymphoma and leukemia. Therapeutic doses of GC induce growth-suppressive and cytotoxic effects on various leukocytes including B cells. Molecular mechanisms of GC action include induction of GC target genes. Glucocorticoid-induced leucine zipper (GILZ) is a rapidly, potently, and invariably GC-induced gene. It mediates a number of GC effects, such as control of cell proliferation, differentiation, and apoptosis. Here we show that deletion of GILZ in mice leads to an accumulation of B lymphocytes in the bone marrow, blood, and lymphoid tissues. Gilz knockout (KO) mice develop a progressive nonlethal B lymphocytosis, with expansion of B220+ cells in the bone marrow and in the periphery, dependent on increased B-cell survival. Decreased B-cell apoptosis in mice lacking GILZ correlates with increased NF-κB transcriptional activity and Bcl-2 expression. B cell–specific gilz KO mice confirmed that the effect of GILZ deletion is B-cell self-intrinsic. These results establish GILZ as an important regulator of B-cell survival and suggest that the deregulation of GILZ expression could be implicated in the pathogenesis of B-cell disorders.
Introduction
Glucocorticoids (GC) are important antiinflammatory/immunosuppressive drugs.1 Their antiinflammatory/immunosuppressive value is the result of the capability to modulate immune cell apoptosis including that of B and T lymphocytes. Notably, GC therapy induces growth-suppressive and cytotoxic effects on various leukocyte types including B cells.2-4
GC have been shown to modulate B-cell proliferation, survival, and differentiation.2,5 These effects lead to a reduction of splenic and lymph nodes B-cell numbers.6 Moreover, many studies of human leukemic lymphoblasts support the hypothesis that GC have preferential apoptotic effects in certain lymphoid cell populations including B-cell lymphoma.1,7,8
Several mechanisms contribute to GC-induced apoptosis and most of the effects mediated by GC depend on the interaction with the GC receptor with consequent modulation of transcriptional activity.9 In particular, GC-induced apoptosis is mediated by transcriptional regulation of Bcl-2 family members, such as downregulation of antiapoptotic protein Bcl-2.10,11 However, the exact mechanisms of GC-mediated programmed cell death are not yet understood.
Among the GC target genes, glucocorticoid-induced leucine zipper (GILZ) is one of the genes most rapidly, potently, and invariably induced by GC treatment.12,13 It mediates a number of GC effects including control of differentiation, cell growth, and apoptosis in several cell types. We have previously shown that GILZ modulates T-lymphocyte differentiation and survival.13,14 GILZ regulates T-helper-cell differentiation15 and mediates GC/transforming growth factor-β signaling during peripheral regulatory T-cell generation.16 Moreover, GILZ has been shown to inhibit cell transformation and growth in a mouse model of RasV12-driven tumorigenesis by suppressing Ras/mitogen-activated protein kinase pathway.17
GILZ inhibits T-cell receptor (TCR)-induced activation of NF-κB transcriptional activity and IL-2/IL-2 receptor expression.18,19 Moreover, GILZ overexpression, consequent to GC treatment, selectively protects from TCR-activated cell death but not from apoptosis induced by other apoptotic stimuli.13 Conversely, GILZ overexpression in thymocytes increases spontaneous apoptosis.20 Notably, GILZ belongs to the TSC22d family, characterized by a leucine zipper motif and by a tsc-box domain; and tsc22d proteins were recently found mutated in diffuse large B-cell lymphoma patients.21
Here we demonstrate that GILZ is expressed in B lymphocytes in different lymphoid tissues including bone marrow (BM), spleen, peripheral lymph nodes (pLN), and in peripheral blood (PB). GILZ expression is evident at different stages of B-cell development and is upregulated by GC treatment. Using mice deleted for gilz gene, we demonstrate that lack of GILZ results in deregulation of B-cell survival starting from the PreB cell stage. We observed increased transcriptional activity of NF-κB, overexpression of Bcl-2 protein and enhanced B-cell survival in gilz knockout (KO) animals. Therefore, GILZ contributes to the control of B-cell apoptosis, and the lack of GILZ results in development of B-cell lymphocytosis.
Methods
Mice
Mice bearing a floxed gilz allele were generated and maintained on a C57Bl/6J background as described previously.22 The conditional gilz KO B-cell animals were obtained by crossing the mice with gilz flox allele with mice CD19-Cre.23 Animal care was in compliance with regulations in Italy (DL 26/2014) and Europe (EU Directive 2010/63/EU).
Quantitative real-time polymerase chain reaction
RNA was isolated using the RNeasy Plus Micro Kit (QIAGEN) and retrotranscribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (qPCR) was performed using the 7300 Real Time PCR System (Applied Biosystems) and the amplifications were done using the TaqMan Gene Expression Master Mix (Applied Biosystem). The qPCR TaqMan probes (Applied Biosystems) used were the following: Bim Mm01333921_m1;Bcl2 Mm00477631_m1;Bmf Mm00506773_m1; Actb 4352341E. For GILZ, the following primers were used:
For: CATGGAGGTGGCGGTCTATC Rev: CACCTCCTCTCTCACAGCGT.
Western blot
Protein extracts were obtained using RIPA buffer supplemented with protease (Sigma-Aldrich) and phosphatase (Thermo Scientific) inhibitor cocktails. Separation of nuclear and cytoplasmic fractions was performed using the Thermo Scientific NE-PER Nuclear and Cytoplasmic Extraction Reagents.
Western blot (WB) analyses were performed with antibody against GILZ (eBioscience), caspase-3 (Cell Signaling Technology), p65NF-kB (Merck Millipore), Bcl-2 (Santa Cruz), laminin (Abcam), and β-tubulin (Sigma-Aldrich) as previously described.16
Antibodies and flow cytometry
For the list of monoclonal antibodies (mAbs) used for flow cytometry analyses and in vitro assays, see supplemental Table 1. Analyses were done using a 2-laser standard configuration ATTUNE NxT (Life Technologies); cell sorting was done using a 2-laser standard configuration fluorescence-activated cell sorting Aria; data were analyzed using FlowJo software (TreeStar). For apoptosis analyses we used CaspACE FITC-VAD-FMK in Situ Marker following the manufacturer’s protocol.
Proximity ligation assay
A Duolink proximity ligation assay was performed according to the manufacturer’s instructions (Olink Bioscience). Anti-GILZ goat (Santa Cruz Biotechnology) and anti-p65 rabbit (Santa Cruz Biotechnology) antibodies were used in blocking buffer overnight at 4°C. Detection of the interaction signals was carried out by fluorescence microscopy using a Zeiss Axioplan fluorescence microscope equipped with a Spot-2 cooled camera (Diagnostic Instruments).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described.16 In brief, cells were fixed in 1% paraformaldehyde and sonicated on ice. Precleared lysates were incubated overnight at 4°C with polyclonal anti-p65 (Santa Cruz Biotechnology) or control rabbit IgG (Cell Signaling Technology). Immunocomplexes were collected using the Chip Assay Kit (Millipore), and qPCR analysis was performed using Power SYBR Green PCR Master Mix (Applied Biosystems). The following primers were used for chip analysis on bcl-2 promoter: for NF-κB binding site: For: CAGAGGAGGGCTTTCTTTCTTCTT; Rev: CCCGGCCTCTTACTTCATTCT. Control region (CR) For: GGGAACAGCACATTCAGTCA; Rev: ACCAACCCACACCATGTACA.
Statistical analysis
Statistical analysis was performed with Prism 6.0 software (GraphPad). The nonparametric Mann-Whitney U test or a 2-tailed unpaired Student t test was used for statistical comparisons (*P < .05, **P < .005, ***P < .0005.).
Results
GILZ is expressed in B lymphocytes
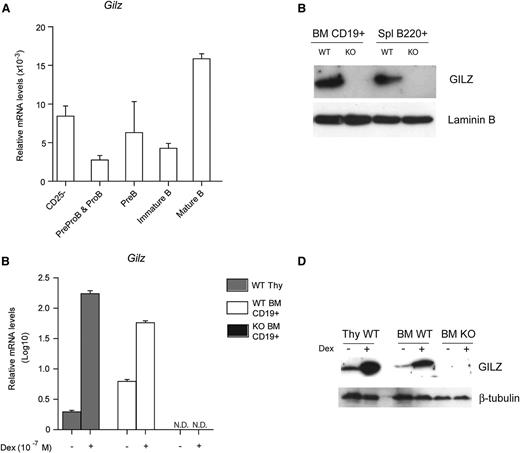
GILZ expression and function have been characterized in many hematopoietic lineages, including T cells, macrophages, and dendritic cells, but its role in B lymphocytes has not been addressed.13,24-27 To determine whether GILZ is expressed in B lymphocytes, BM cells from WT mice were analyzed by qPCR for the expression of GILZ at various stages of B-cell development (Figure 1A). We have found that GILZ is expressed in all B-cell developmental stages at levels comparable with those of CD4+CD25– T cells, with the highest expression levels of GILZ mRNA detected in mature/recirculating IgM+IgD+ B cells (Figure 1A). GILZ is expressed in purified B cells from BM and spleen as assessed by WB analysis (Figure 1B).
GILZ is expressed in B lymphocytes and is upregulated by GC treatment. (A) qPCR analysis of Gilz mRNA expression in WT naïve T cells and in sorted B cells subpopulation from BM (as described in supplemental Methods) (n = 3). (B) WB analysis of GILZ expression in bone marrow (BM) CD19+ cells or in spleen (Spl) B220+ cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with β-laminin antibodies served as loading control. (C) qPCR analysis of Gilz mRNA expression in WT thymus (Thy) cells and in CD19+ cells isolated from the BM of WT and KO mice cultured for 4 hours with or without Dex 10−7 M (n = 3). N.D., not detectable. Data in (A) and (C) were derived from 2 independent experiments and are presented relative to the expression of Actb mRNA. (D) WB analysis of GILZ expression in WT Thy cells and in BM cells from WT and gilz KO mice cultured for 4 hours with or without Dex 10−7 M. The same number of cells was loaded; WB with β-laminin antibodies served as loading control. Graphs represent mean ± standard error of the mean (SEM).
GILZ is expressed in B lymphocytes and is upregulated by GC treatment. (A) qPCR analysis of Gilz mRNA expression in WT naïve T cells and in sorted B cells subpopulation from BM (as described in supplemental Methods) (n = 3). (B) WB analysis of GILZ expression in bone marrow (BM) CD19+ cells or in spleen (Spl) B220+ cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with β-laminin antibodies served as loading control. (C) qPCR analysis of Gilz mRNA expression in WT thymus (Thy) cells and in CD19+ cells isolated from the BM of WT and KO mice cultured for 4 hours with or without Dex 10−7 M (n = 3). N.D., not detectable. Data in (A) and (C) were derived from 2 independent experiments and are presented relative to the expression of Actb mRNA. (D) WB analysis of GILZ expression in WT Thy cells and in BM cells from WT and gilz KO mice cultured for 4 hours with or without Dex 10−7 M. The same number of cells was loaded; WB with β-laminin antibodies served as loading control. Graphs represent mean ± standard error of the mean (SEM).
Because GILZ is a GC-inducible gene, we investigated whether GC transcriptionally increase GILZ levels in B cells. Figure 1C indicates that dexamethasone (Dex) treatment upregulates mRNA and protein GILZ levels in purified CD19+ B cells, as it occurs in thymocytes14 (Figure 1C-D). These data indicate that GILZ is expressed constitutively in B cells and that its expression is upregulated by GC treatment.
GILZ regulates B-cell homeostasis
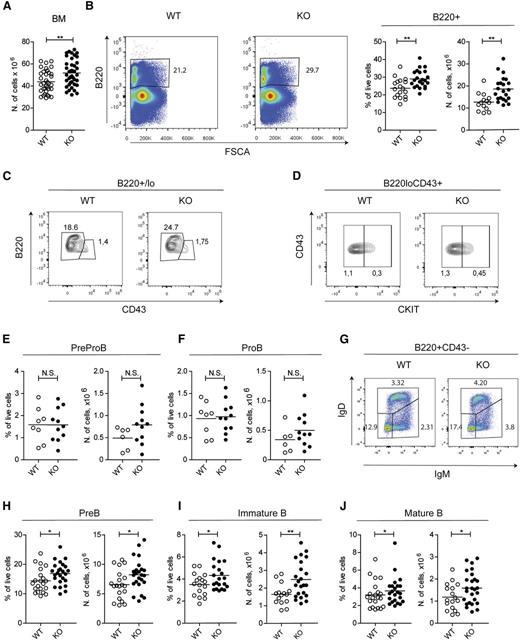
To address the role of GILZ in B-cell homeostasis, we have used the genetic approach. Mice deleted for the gilz gene were recently generated.22 Young gilz KO mice showed normal body and lymphoid tissue weight (supplemental Figure 1A-B) and absolute counts of white blood cells (supplemental Figure 1C), as well as B and T lymphocytes (supplemental Figure 1D-E). However, the total number of BM cells was significantly higher in gilz KO mice compared with controls (Figure 2A). Flow cytometry analysis of BM revealed an increased frequency and number of B220+ cells in gilz KO mice compared with WT controls (Figure 2B), whereas development of other hematologic lineages appeared normal (supplemental Figure 1D). To further characterize the effect of gilz deficiency in B-cell lineage, we have analyzed the frequency and number of B-cell subpopulations at specific stages of B-cell development. Flow cytometry analysis showed that Pre-ProB (B220loCD43+cKit–) and ProB cell fractions (B220loCD43+cKit+) were similarly present in the BM of WT and gilz KO mice (Figure 2C-F), whereas gilz KO mice revealed a mild but significant increase in the frequency and absolute number of PreB (B220+CD43–IgM–IgD–), immature B (B220+CD43–IgM+IgD–), and mature/recirculating B cells (B220+CD43–IgM+IgD+) (Figure 2G-J). Altogether these data indicate that GILZ is dispensable for the very early stages of B-cell development, whereas it is important for B-cell homeostasis starting from the PreB stage.
GILZ regulates B-cell homeostasis in BM. (A) Number of cells in BM of 8- to 12-week-old WT and gilz KO mice. (B) Flow cytometry analysis of B220 expression (left) in cells derived from BM of 8- to 12-week-old WT and gilz KO mice. On the right are the frequency and number of B220+ cells in BM isolated from 8- to 12-week-old WT and gilz KO mice. (C) Flow cytometry analysis of B220 and CD43 expression in B220+/lo cells of BM of 8- to 12-week-old WT and gilz KO mice. (D) Flow cytometry analysis of cKIT expression in B220loCD43+ cells of BM of 8- to 12-week-old WT and gilz KO mice. (E) Frequency and number of PreProB (B220loCD43+cKIT–), or ProB (B220loCD43+cKIT+) (F). (G) Flow cytometry analysis of IgM and IgD expression in B220+CD43– cells of BM of 8- to 12-week-old WT and gilz KO mice. (H-J) Frequency and number of PreB cells (B220+CD43–IgM–IgD–) (H), immature B cells (B220+CD43–IgM+IgD–) (I), or mature B cells (B220+CD43–IgM−/+IgD+) (J) isolated from 8- to 12-week-old WT and gilz KO mice. Each symbol represents an individual mouse; small horizontal lines indicate the mean. Data were derived from 7 independent experiments. *P < .05, **P < .005, ***P < .0005.
GILZ regulates B-cell homeostasis in BM. (A) Number of cells in BM of 8- to 12-week-old WT and gilz KO mice. (B) Flow cytometry analysis of B220 expression (left) in cells derived from BM of 8- to 12-week-old WT and gilz KO mice. On the right are the frequency and number of B220+ cells in BM isolated from 8- to 12-week-old WT and gilz KO mice. (C) Flow cytometry analysis of B220 and CD43 expression in B220+/lo cells of BM of 8- to 12-week-old WT and gilz KO mice. (D) Flow cytometry analysis of cKIT expression in B220loCD43+ cells of BM of 8- to 12-week-old WT and gilz KO mice. (E) Frequency and number of PreProB (B220loCD43+cKIT–), or ProB (B220loCD43+cKIT+) (F). (G) Flow cytometry analysis of IgM and IgD expression in B220+CD43– cells of BM of 8- to 12-week-old WT and gilz KO mice. (H-J) Frequency and number of PreB cells (B220+CD43–IgM–IgD–) (H), immature B cells (B220+CD43–IgM+IgD–) (I), or mature B cells (B220+CD43–IgM−/+IgD+) (J) isolated from 8- to 12-week-old WT and gilz KO mice. Each symbol represents an individual mouse; small horizontal lines indicate the mean. Data were derived from 7 independent experiments. *P < .05, **P < .005, ***P < .0005.
GILZ-deficient mice develop B-cell lymphocytosis
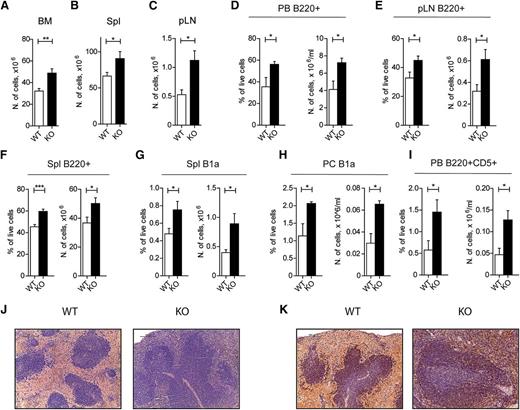
Because GILZ was shown to suppress tumor cell growth,17 and because the tsc22d family members were found to be mutated in B-cell lymphoma patients,21 we followed gilz KO animals for development of leukemia/lymphoma overtime. White blood cell (WBC) and lymphocyte counts were significantly increased in aged gilz-KO mice (13-19 months old) compared with age-matched controls. Lymphocytosis developing in gilz KO mice was characterized by an accumulation of lymphocytes in the PB (about a twofold increase; Table 1). Moreover, we observed increased cellularity of BM (Figure 3A), spleen (Figure 3B), and pLN (Figure 3C) in gilz KO mice compared with WT littermates. The increased lymphocyte number was the result of a specific accumulation of B220+ B cells in mice lacking GILZ, as is shown in PB (Figure 3D) and peripheral lymphoid organs (Figure 3E-F), including CD5+ B1a splenic and peritoneal cells (Figure 3G-H) and B220+CD5+ cells in PB (Figure 3I). Conversely, the number of CD4+, CD8+, and Mac-1+ cells did not differ in respective organs of WT and gilz KO mice (supplemental Figure 2). Morphologic analysis of the spleen of gilz KO mice shows that the area occupied by the white pulp is reduced to the important presence of small lymphocytes spread beyond the follicular area (Figure 3J), with a significant increased number of B220+ cells in the extrafollicular regions (Figure 3K).
Complete blood counts in aged WT and gilz KO mice (20-24 months old)
| . | Wild-type . | gilz knockout . |
|---|---|---|
| WBC | 10.8 ± 2.23 | 18.94 ± 4.85* |
| NE | 3.02 ± 1.70 | 4.7 ± 1.29 |
| Ly | 7.10 ± 2.66 | 13.26 ± 3.94* |
| MO | 0.48 ± 0.36 | 0.71 ± 0.25 |
| EO | 0.10 ± 0.05 | 0.23 ± 0.18 |
| BA | 0.01 ± 0.01 | 0.034 ± 0.05 |
| RBC | 8.83 ± 0.61 | 8.78 ± 1.25 |
| Hb | 12.27 ± 0.64 | 11.92 ± 1.79 |
| HT | 41.92 ± 2.60 | 41.42 ± 5.62 |
| MCV | 53.71 ± 3.79 | 53.86 ± 3.82 |
| MCH | 15.76 ± 1.25 | 15.47 ± 1.31 |
| MCHC | 66.12 ± 89.51 | 32.74 ± 2.64 |
| RDW | 20.89 ± 1.18 | 22.39 ± 2.85 |
| PLT | 1306.38 ± 351.93 | 1388.25 ± 299.58 |
| MPV | 6.01 ± 0.55 | 6.01 ± 0.67 |
| . | Wild-type . | gilz knockout . |
|---|---|---|
| WBC | 10.8 ± 2.23 | 18.94 ± 4.85* |
| NE | 3.02 ± 1.70 | 4.7 ± 1.29 |
| Ly | 7.10 ± 2.66 | 13.26 ± 3.94* |
| MO | 0.48 ± 0.36 | 0.71 ± 0.25 |
| EO | 0.10 ± 0.05 | 0.23 ± 0.18 |
| BA | 0.01 ± 0.01 | 0.034 ± 0.05 |
| RBC | 8.83 ± 0.61 | 8.78 ± 1.25 |
| Hb | 12.27 ± 0.64 | 11.92 ± 1.79 |
| HT | 41.92 ± 2.60 | 41.42 ± 5.62 |
| MCV | 53.71 ± 3.79 | 53.86 ± 3.82 |
| MCH | 15.76 ± 1.25 | 15.47 ± 1.31 |
| MCHC | 66.12 ± 89.51 | 32.74 ± 2.64 |
| RDW | 20.89 ± 1.18 | 22.39 ± 2.85 |
| PLT | 1306.38 ± 351.93 | 1388.25 ± 299.58 |
| MPV | 6.01 ± 0.55 | 6.01 ± 0.67 |
BA, basophils; EO, eosinophil count; Hb, hemoglobin; HT, hematocrit; Ly, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; MCV, mean corpuscular volume; MO, monocytes; NE, neutrophils; PLT, platelets; RBC, red blood cells; RDW, RBC distribution width; WBC, white blood cells.
Wild-type, n = 9; gilz knockout, n = 7.
P < .005.
Old GILZ KO mice develop a chronic B-cell lymphocytosis. (A-C) Number of cells in BM (A), spleen (B), and pLN (C) isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (D) Frequency (left) and number (right) of B220+ cells in PB isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (E) Frequency (left) and number (right) of B220+ cells in pLN isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (F) Frequency (left) and number (right) of B220+ cells in spleen isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (G) Frequency (left) and number (right) of B1a (B220loIgM+IgD–CD43+CD5+) cells in spleen isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (H) Frequency (left) and number (right) of B1a cells in peritoneum (PC) isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (I) Frequency (left) and number (right) of B220+CD5+ cells in blood isolated from 13- to 19-month-old WT and gilz KO mice (n = 4). (J) Spleen histologic phenotype of 18-month-old WT and gilz KO mice assessed by hematoxylin and eosin (H&E) staining. Scale bars represent 100 µm; original magnification ×20. (K) Immunohistochemical analyses show that the number of CD45R/B220+ lymphocytes in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20.
Old GILZ KO mice develop a chronic B-cell lymphocytosis. (A-C) Number of cells in BM (A), spleen (B), and pLN (C) isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (D) Frequency (left) and number (right) of B220+ cells in PB isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (E) Frequency (left) and number (right) of B220+ cells in pLN isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (F) Frequency (left) and number (right) of B220+ cells in spleen isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (G) Frequency (left) and number (right) of B1a (B220loIgM+IgD–CD43+CD5+) cells in spleen isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (H) Frequency (left) and number (right) of B1a cells in peritoneum (PC) isolated from 13- to 19-month-old WT and gilz KO mice (n = 9/10). (I) Frequency (left) and number (right) of B220+CD5+ cells in blood isolated from 13- to 19-month-old WT and gilz KO mice (n = 4). (J) Spleen histologic phenotype of 18-month-old WT and gilz KO mice assessed by hematoxylin and eosin (H&E) staining. Scale bars represent 100 µm; original magnification ×20. (K) Immunohistochemical analyses show that the number of CD45R/B220+ lymphocytes in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20.
To investigate whether production of immunoglobulin (Ig) was increased in gilz KO mice, we measured serum Ig levels in aged WT and gilz KO animals. Results show slight but not statistically significant increased production of IgG1, IgG2, IgE, IgM, and antinuclear antibody (ANA) (supplemental Figure 3A) as well as comparable frequency and number of B220–/loCD138hi plasma cell in GILZ-deficient mice compared with controls (supplemental Figure 3B-C), suggesting that no clear signs of auto-iummunity are evident in gilz-deficient mice.
We also investigated whether the expansion of B cells in gilz KO animals was monoclonal, because cancers evolve by a reiterative process of clonal expansion. Monoclonal B-cell lymphocytosis is a well-characterized condition that displays biological similarities to the most common adult chronic lymphocytic leukemia.28-30 Gilz KO B cells, purified from PB or spleen, did not show signs of clonality as assessed by qPCR amplification and sequencing of endogenous rearranged Ig heavy-chain genes (JH intron) (supplemental Figure 4). The peripheral accumulation of gilz KO B cells was not associated with premature lethality or morbidity of gilz KO mice (data not shown). Altogether, these data demonstrate that lack of GILZ results in a chronic nonlethal B-cell disorder.
The effect of GILZ on B cells is self-intrinsic
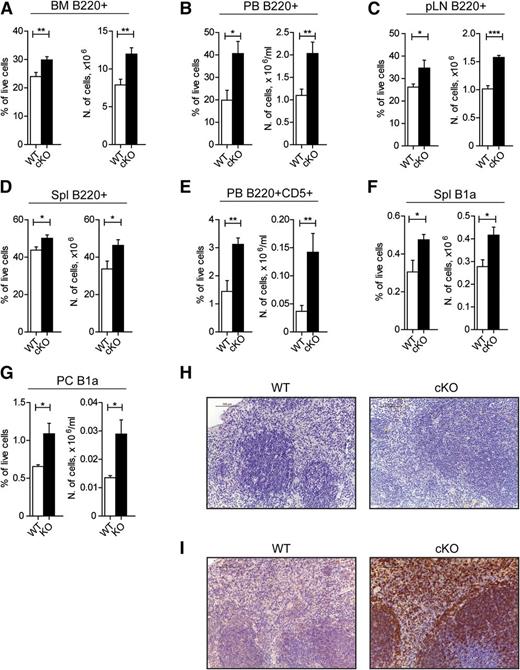
To demonstrate that the observed defect was B cell–specific, we have analyzed the effect of GILZ deletion in gilz “floxed” mice crossed with the transgenic mice bearing Cre recombinase under the CD19 promoter (hereafter referred to as gilz CD19-KO mice) that show Cre-mediated LoxP recombination only in B cells23 (supplemental Figure 5A). Similar to what is observed in gilz KO mice, frequency and number of B220+ cells were higher in the BM of gilz CD19-KO mice (Figure 4A). Analysis of B-cell subsets showed that the B-cell accumulation in gilz CD19-KO mice also starts at the PreB stage of B-cell development (supplemental Figure 5C), demonstrating that the B-cell defect in GILZ-deficient mice is self-intrinsic. Interestingly, gilz CD19-KO mice showed an earlier accumulation of B cells in the periphery (Figure 4B-D), compared with gilz KO mice. Complete analysis of peripheral B-cell subsets revealed an increased number of B cells in the spleen of 8- to 12-week-old gilz CD19-KO mice, although the frequency of immature B, marginal B, and follicular B-cell subsets was unaltered (supplemental Figure 5D). Similarly to what is observed in aged gilz KO mice, B220+CD5+ cells were increased in PB (Figure 4E), and the number of B1a B cells increased in the spleen and peritoneal cavity of gilz CD19-KO mice compared with WT controls (Figure 4F-G, respectively), proportional to the overall increase in B220+ cells. Moreover, histologic analyses of spleen isolated from GILZ CD19-KO mice (Figure 4H-I) reveal a significant increased number of B220+ cells in extrafollicular regions, similar to what is observed in aged gilz KO mice (Figure 4H-I). Despite an earlier onset of B-cell lymphocytosis, gilz CD19-KO mice did not reveal any signs of frank leukemia or autoimmune disease at 8 to 9 months of age, as evidenced by histologic analysis of several tissues (data not shown), by peripheral blood WBC counts (supplemental Figure 5E), as well as by lack of differences in frequency and number of plasma cells and Ig production in serum of WT and gilz CD19-KO mice (supplemental Figure 3A-C).
Lack of GILZ develops lymphocytosis in a B-cell autonomous fashion. (A-D) Frequency (left) and number (right) of B220+ cells in BM (A), PB (B), pLN (C), and Spl (D) isolated from 8- to 12-week-old WT and gilz CD19-KO mice (n = 7/8). (E) Frequency (left) and number (right) of B220+CD5+ cells in PB isolated from 8- to 12-week-old WT and gilz cKO mice (n = 7/8). (F-G) Frequency (on left) and number (on right) of B1a cells in spleen (F) or PC (G) isolated from 8- to 12-week-old WT and gilz cKO mice (n = 6/8). (H) Spleen histologic phenotype of 8- to 12-week-old WT and gilz KO mice assessed by H&E staining. Scale bars represent 100 µm; original magnification ×20. (I) Immunohistochemical analyses show that the number of CD45R/B220+ lymphocytes in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20. Graphs represent mean ± SEM. Data are from 2 independent experiments. *P < .05, **P < .005, ***P < .0005.
Lack of GILZ develops lymphocytosis in a B-cell autonomous fashion. (A-D) Frequency (left) and number (right) of B220+ cells in BM (A), PB (B), pLN (C), and Spl (D) isolated from 8- to 12-week-old WT and gilz CD19-KO mice (n = 7/8). (E) Frequency (left) and number (right) of B220+CD5+ cells in PB isolated from 8- to 12-week-old WT and gilz cKO mice (n = 7/8). (F-G) Frequency (on left) and number (on right) of B1a cells in spleen (F) or PC (G) isolated from 8- to 12-week-old WT and gilz cKO mice (n = 6/8). (H) Spleen histologic phenotype of 8- to 12-week-old WT and gilz KO mice assessed by H&E staining. Scale bars represent 100 µm; original magnification ×20. (I) Immunohistochemical analyses show that the number of CD45R/B220+ lymphocytes in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20. Graphs represent mean ± SEM. Data are from 2 independent experiments. *P < .05, **P < .005, ***P < .0005.
These data demonstrate that the effect of GILZ deletion is mainly B cell–intrinsic and that lack of GILZ in B cells results in indolent B lymphocytosis, similar to what was observed in aged gilz KO mice.
Lack of GILZ inhibits spontaneous B-cell apoptosis
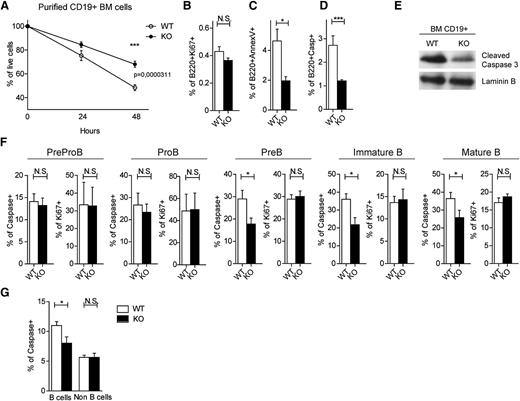
To investigate whether the B-lymphocyte accumulation is a result of a decreased cell death, we tested the ability of WT and gilz KO B cells to survive in vitro. Purified CD19+ cells lacking GILZ showed increased survival in vitro compared with WT B cells, as measured by cell counting after 48 hours in culture (Figure 5A). Interestingly, the proliferation rate of WT and gilz KO B cells was comparable (Figure 5B), as revealed by a similar expression of the proliferation marker ki67. Moreover, to test whether increased B-cell counts were caused by a decreased spontaneous apoptosis, we compared apoptosis in WT and GILZ-deficient B cells by Annexin V staining. Results demonstrate that cells lacking GILZ have a decreased frequency of apoptotic cells in BM (Figure 5C) and spleen (supplemental Figure 6). Consistently, GILZ-deficient cells show decreased activation of caspases, downstream effectors of apoptosis (Figure 5D-E). These results demonstrate that GILZ deficiency leads to a decreased apoptosis of B cells in vitro.
Lack of GILZ enhances B-cell survival. (A) Percentage of live cells of BM CD19+ cells from WT and gilz KO mice after 24 or 48 hours in vitro (n = 4/5). (B-D) Frequency of B220+Ki67+ cells (B), B220+AnnexinV+ cells (C), and B220+Caspase+ (D) isolated from the BM of WT and gilz KO mice after 48 hours in vitro (n = 4/5). (E) WB analysis of cleaved caspase 3 expression in BM CD19+ cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with laminin B antibodies served as loading control. (F) Frequency of Caspase+ cells and Ki67+ on PreProB cells, ProB cells, PreB cells, immature B cells, and mature B cells isolated from the BM of WT and KO mice (n = 10/12). (G) Frequency of Caspase+ on B cells (B220+) and non–B cells (B220–) from BM isolated from WT and gilz KO mice (n = 10/12). *P < .05, *P < .05, **P < .005, ***P < .0005.
Lack of GILZ enhances B-cell survival. (A) Percentage of live cells of BM CD19+ cells from WT and gilz KO mice after 24 or 48 hours in vitro (n = 4/5). (B-D) Frequency of B220+Ki67+ cells (B), B220+AnnexinV+ cells (C), and B220+Caspase+ (D) isolated from the BM of WT and gilz KO mice after 48 hours in vitro (n = 4/5). (E) WB analysis of cleaved caspase 3 expression in BM CD19+ cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with laminin B antibodies served as loading control. (F) Frequency of Caspase+ cells and Ki67+ on PreProB cells, ProB cells, PreB cells, immature B cells, and mature B cells isolated from the BM of WT and KO mice (n = 10/12). (G) Frequency of Caspase+ on B cells (B220+) and non–B cells (B220–) from BM isolated from WT and gilz KO mice (n = 10/12). *P < .05, *P < .05, **P < .005, ***P < .0005.
The major resistance of gilz KO B cells to spontaneous death in vitro correlates with the increased number of B220+ cells in gilz KO mice (Figure 2B). To determine whether increased B-cell numbers in the BM of gilz KO mice cells was caused by a decreased apoptosis in vivo, we evaluated cell proliferation and death in different B-cell subpopulations ex vivo by flow cytometry. Expression of ki67 was comparable in WT and gilz KO mice in all of the B-cell subsets, suggesting that B-cell accumulation in gilz KO mice is not a result of an increased proliferation (Figure 5F). Instead, B cells isolated from gilz KO mice revealed decreased caspase activation in all B-cell subsets starting from the PreB stage of B development (Figure 5F). Interestingly, the non–B cells fraction of the BM showed a similar level of caspase activation (Figure 5G). Altogether, these data demonstrate that lack of GILZ results in a defective apoptosis specifically in B cells.
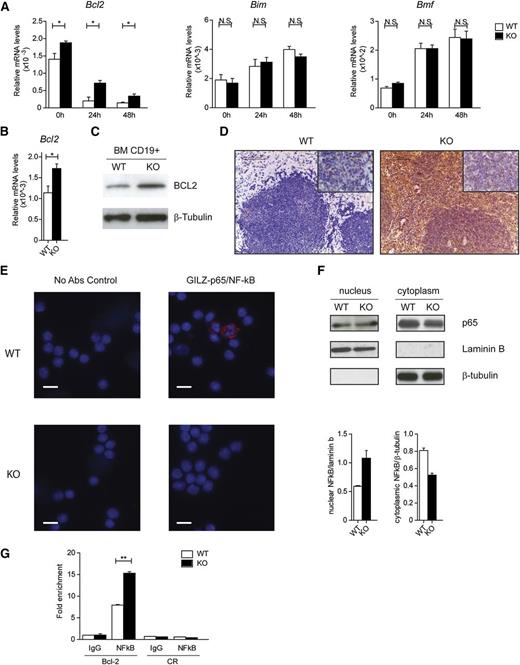
To define the mechanism by which GILZ controls cell survival, we monitored the expression levels of different Bcl-2 family members in cultured BM cells isolated from WT and gilz KO mice. Purified CD19+ BM cells lacking GILZ exhibited a significant increase in Bcl-2 expression compared with WT B cells, when cultured in vitro (Figure 6A, left panel), whereas expression levels of other Bcl-2 family members that regulate B-cell apoptosis, such as Bim and Bmf31 (Figure 6A, middle and right panels), were unaltered. Deregulation of Bcl-2 expression in gilz-deficient cells was confirmed both at the mRNA (Figure 6B) and protein levels (Figure 6C) in purified CD19+ cells analyzed ex vivo, and was also evidenced in the spleens of aged gilz KO mice by immunohistochemistry analysis (Figure 6D).
Lack of GILZ enhances NF-κB transcriptional activity and Bcl-2 expression. (A) qPCR analysis of Bcl2, Bim, and Bmf mRNA expression in CD19+ BM cells cultured in vitro for the time indicated in the graph. (B) qPCR analysis of Bcl2 mRNA expression in CD19+ cells isolated from the BM of WT and KO mice (n = 6). Data are presented relative to the expression of Actb mRNA. (C) WB analysis of Bcl2 expression in purified B cells isolated from WT and gilz KO mice. (D) Immunohistochemical analyses show that the number of Bcl-2+ cells in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20. Insets in the upper right image represent higher magnification images (40×). (E) Images of in situ proximity ligation assay (PLA) performed on CD19+ cells isolated from the BM of WT mice. PLA was carried out in the absence (left) or presence (right) GILZ-p65/NF-κB primary antibodies mix. The right panel shows a representative merge of the red (PLA) and blue nuclear counterstain (DAPI) channels images. The red spots indicate close proximity (<40 nm) between bound antibodies. Scale bars represent 20 µm. (F) WB analysis of p65 expression in purified B cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with β-tubulin and laminin B antibodies served as loading control. The graph below represents densitometric analysis of NF-κB expression relative to housekeeping controls (n = 2). (G) Chromatin IP assay of NF-κB binding on proximal promoter of Bcl2 in WT and gilz KO CD19+ cells isolated from BM. Cell lysates were immunoprecipitated with anti–NF-κB or control IgG, and the presence of specific regions in the immunoprecipitates was determined by qPCR. Graphs represent mean ± SEM. *P < .05, **P < .005, ***P < .0005.
Lack of GILZ enhances NF-κB transcriptional activity and Bcl-2 expression. (A) qPCR analysis of Bcl2, Bim, and Bmf mRNA expression in CD19+ BM cells cultured in vitro for the time indicated in the graph. (B) qPCR analysis of Bcl2 mRNA expression in CD19+ cells isolated from the BM of WT and KO mice (n = 6). Data are presented relative to the expression of Actb mRNA. (C) WB analysis of Bcl2 expression in purified B cells isolated from WT and gilz KO mice. (D) Immunohistochemical analyses show that the number of Bcl-2+ cells in spleen is increased in gilz KO compared with WT mice. Scale bars represent 100 µm; original magnification ×20. Insets in the upper right image represent higher magnification images (40×). (E) Images of in situ proximity ligation assay (PLA) performed on CD19+ cells isolated from the BM of WT mice. PLA was carried out in the absence (left) or presence (right) GILZ-p65/NF-κB primary antibodies mix. The right panel shows a representative merge of the red (PLA) and blue nuclear counterstain (DAPI) channels images. The red spots indicate close proximity (<40 nm) between bound antibodies. Scale bars represent 20 µm. (F) WB analysis of p65 expression in purified B cells isolated from WT and gilz KO mice. The same number of cells was loaded; WB with β-tubulin and laminin B antibodies served as loading control. The graph below represents densitometric analysis of NF-κB expression relative to housekeeping controls (n = 2). (G) Chromatin IP assay of NF-κB binding on proximal promoter of Bcl2 in WT and gilz KO CD19+ cells isolated from BM. Cell lysates were immunoprecipitated with anti–NF-κB or control IgG, and the presence of specific regions in the immunoprecipitates was determined by qPCR. Graphs represent mean ± SEM. *P < .05, **P < .005, ***P < .0005.
Among the pathways known to be regulated by GILZ and control apoptosis in B cells, NF-κB activation is pivotal for modulation of Bcl-2 family members.32 We and others have previously demonstrated that GILZ directly binds to and represses NF-κB transcriptional activity in T lymphocytes, macrophages, and other cell types.18,19,25,33,34 Here we found that GILZ interacts with the NF-κB p65 subunit in purified CD19+ B cells as revealed by proximity ligation assay (Figure 6E, top right panel), suggesting that GILZ regulates NF-κB also in B cells. Consistently, we observed an increased nuclear translocation of the NF-κB p65 subunit in gilz-deficient B cells (Figure 6F). To test whether lack of GILZ leads to an increased NF-κB transcriptional activity at the Bcl-2 promoter, we performed ChIP assay in CD19+ B cells purified from WT or gilz KO animals. Results indicate that p65/NF-κB binds more to the Bcl-2 promoter in purified B cells lacking GILZ (Figure 6G). Of note, other pathways inhibited by GILZ in other cell types, such as ERK and Akt,18,35 were not enhanced in gilz-deficient B cells, stimulated or not by anti-IgM antibody, compared with controls (supplemental Figure 7). These data indicate that lack of GILZ results in increased NF-κB nuclear translocation and transcriptional activity, leading to an increase in Bcl-2 expression and B-cell survival.
GILZ deficiency precludes GC-mediated B-cell apoptosis
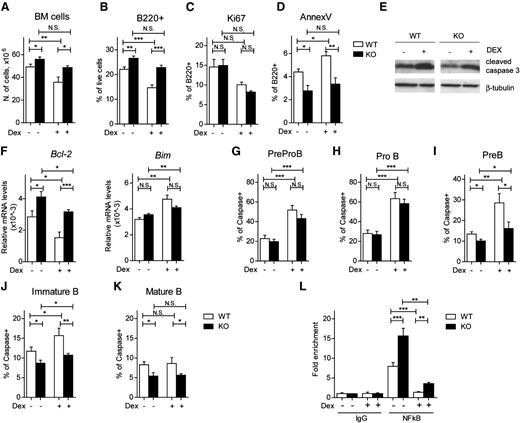
GC treatment is associated with a highly effective induction of apoptosis in lymphocytes, including B cells.36 GILZ has been shown to be an effector of various GC effects,17,25,37-40 including apoptosis.13,20 To evaluate the possible role of GILZ in GC-induced apoptosis, we compared BM counts in WT and gilz KO mice treated in vivo with Dex. Figure 7A shows that Dex-mediated reduction in BM cell numbers observed in WT mice is significantly lower in mice lacking GILZ, suggesting that GILZ plays a specific role in GC-induced apoptosis. We then tested whether this BM cell reduction was a result of the GC effect on B lymphocytes. Flow cytometry analysis of B220+ cells revealed, as expected, that Dex reduced the number of B cells in WT mice, whereas this reduction was significantly less pronounced in mice lacking GILZ (Figure 7B). The differences in B-cell number in Dex-treated animals were not because of a lack of GC-induced growth arrest, because the frequency of Ki67+ cells among B220+ cells was similar in GC-treated WT and gilz KO cells (Figure 7C). Instead, GILZ-deficient B cells showed decreased Dex-induced apoptosis compared with WT cells, as revealed by AnnexinV staining (Figure 7D) and western blot analysis of caspase activation of B cells isolated from Dex-treated WT and gilz KO mice (Figure 7E). Furthermore, analysis of caspase activation in different B-cell subsets revealed that lack of GILZ did not affect Dex-induced cell death in Pre-ProB and ProB cells (Figure 7G-H), whereas it partially rescued apoptosis in PreB and immature B cells. (Figure 7I-J, respectively). Finally, GILZ exerted proapoptotic effects in mature B cells, resistant to pharmacologic doses of GC (Figure 7K). These results are consistent with data showing that GILZ regulates B-cell survival starting at the PreB stage of differentiation (Figure 2E-J). These data suggest that lack of GILZ partially rescued Dex-induced apoptosis in PreB and immature B cells, whereas it was not sufficient to protect from Dex-induced apoptosis at earlier stages of B-cell differentiation.
GILZ mediates GC-induced B-cell apoptosis. (A) Number of cells in the BM of 8- to 12-week-old WT and gilz KO mice untreated or treated with Dex 3 mg/kg intraperitoneally (i.p.) for 3 days (n = 5/7). (B-D) Frequency of B220+ cells (B), B220+Ki67+ cells (C) and B220+AnnexV+ cells (D) isolated from the BM of WT and KO mice untreated or treated with Dex 3 mg/kg i.p. for 3 days (n = 5/7). (E) WB analysis of cleaved caspase 3 expression in purified B cells isolated from WT and gilz KO mice treated or not with Dex. The same number of cells was loaded; WB with β-tubulin antibodies served as loading control. (F) qPCR analysis of Bcl2 and Bim mRNA expression in purified B cells isolated from WT and gilz KO mice treated or not with Dex (n = 8/10). (G-K) Frequency of Caspase+ cells on PreProB cells (G), ProB cells (H), PreB cells (I), immature B cells (J), and mature B cells (K) isolated from BM of WT and KO mice untreated or treated with Dex (n = 9/12). (L) Chromatin IP assay of NF-κB binding on proximal promoter of Bcl2 in purified B cells of WT and gilz KO mice treated or not with Dex. Cell lysates were immunoprecipitated with anti–NF-κB or control IgG, and the presence of specific regions in the immunoprecipitates was determined by qPCR. CR indicates qPCR in a negative control region 2 kb upstream of the NF-κB binding site. Graphs represent mean ± SEM. Data are from 2 independent experiments. *P < .05, **P < .005, ***P < .0005.
GILZ mediates GC-induced B-cell apoptosis. (A) Number of cells in the BM of 8- to 12-week-old WT and gilz KO mice untreated or treated with Dex 3 mg/kg intraperitoneally (i.p.) for 3 days (n = 5/7). (B-D) Frequency of B220+ cells (B), B220+Ki67+ cells (C) and B220+AnnexV+ cells (D) isolated from the BM of WT and KO mice untreated or treated with Dex 3 mg/kg i.p. for 3 days (n = 5/7). (E) WB analysis of cleaved caspase 3 expression in purified B cells isolated from WT and gilz KO mice treated or not with Dex. The same number of cells was loaded; WB with β-tubulin antibodies served as loading control. (F) qPCR analysis of Bcl2 and Bim mRNA expression in purified B cells isolated from WT and gilz KO mice treated or not with Dex (n = 8/10). (G-K) Frequency of Caspase+ cells on PreProB cells (G), ProB cells (H), PreB cells (I), immature B cells (J), and mature B cells (K) isolated from BM of WT and KO mice untreated or treated with Dex (n = 9/12). (L) Chromatin IP assay of NF-κB binding on proximal promoter of Bcl2 in purified B cells of WT and gilz KO mice treated or not with Dex. Cell lysates were immunoprecipitated with anti–NF-κB or control IgG, and the presence of specific regions in the immunoprecipitates was determined by qPCR. CR indicates qPCR in a negative control region 2 kb upstream of the NF-κB binding site. Graphs represent mean ± SEM. Data are from 2 independent experiments. *P < .05, **P < .005, ***P < .0005.
To investigate whether Bcl-2 deregulation may also contribute to protection from Dex-induced apoptosis, we have assessed Bcl-2 expression in B cells after Dex treatment. Dex is able to activate a wide number of molecular mechanisms possibly involved in apoptosis regulation, including Bcl-2 levels of regulation. We here show that Dex treatment led to a decrease in Bcl-2 expression levels in WT B cells (45% reduction Dex-treated vs untreated), whereas this decrease was smaller (23% reduction) in gilz KO cells (Figure 7F), suggesting that GILZ contributes to GC-mediated effects. Alternatively, Dex decreases NF-κB presence at the Bcl-2 promoter both in WT cells (83% reduction) and in gilz KO cells (77% reduction) (Figure 7L). Altogether, these data point to a role of GILZ in mediating the effects of GC on B-cell apoptosis and indicate that, different from untreated WT and gilz KO cells, mechanisms other than NF-κB–dependent regulation on Bcl-2 expression are involved in Dex-induced apoptosis.
Discussion
In this manuscript we demonstrate that GILZ plays an important role in the control of B-cell apoptosis. Lack of GILZ results in accumulation of B220+ cells in BM and peripheral lymphoid organs and it is associated with increased B-cell survival.
We generated and aged cohorts of WT and gilz KO mice to evaluate their spontaneous predisposition to cancer or autoimmunity caused by GILZ deficiency. We found that old gilz KO mice develop B-cell lymphocytosis (twofold increase in B220+ cell numbers higher than controls). The B-cell accumulation observed in gilz KO mice seems to take origin in BM. In fact, young mice show a normal hematologic profile in PB and lymphoid organs, whereas BM counts and B220+ cells are significantly augmented compared with age-matched WT controls. B-cell accumulation becomes then evident in periphery with age and leads to lymphocytosis. Notably, the spleen of aged mice displayed accumulation of small B cells in the splenic extrafollicular region. Of note, all peripheral B-cell subsets, including immature, follicular, marginal, and B1a B cells, were proportionally increased in gilz-deficient mice. Altogether, the major defect observed in aged gilz KO mice is a chronic nonlethal B lymphocytosis, without any clear signs of pathology at steady state, which may predispose to B cell–mediated disorders.
To demonstrate that the defect of gilz deficiency is B cell–intrinsic, we carried out experiments with selective gilz ablation in B-cell lineage using conditional gilz CD19-KO mice. Results indicate that the B-cell defect in gilz KO mice is cell intrinsic, because mice with B cell–restricted gilz deletion show increased B cells in the BM and periphery. CD-19 KO and the gilz KO mice both reveal the same phenotype in the B-cell compartment but with different kinetics, because gilz CD19-KO mice developed B-cell lymphocytosis at a younger age. We cannot exclude that GILZ deficiency in other non–B cell types (eg, T cell and stromal cells) in gilz KO mice dampens the B cell–intrinsic defect. However, the major defect observed is a chronic B lymphocytosis, without any clear signs of frank B-cell lymphoma or autoimmune diseases in both models over time. Altogether, results indicate that the defect in B-cell homeostasis in gilz KO mice is B cell–intrinsic.
Our data suggest that the underlying cause of B-cell accumulation observed in aged gilz KO mice is a defective B-cell apoptosis rather than deregulated cell proliferation. We found that decreased apoptosis correlates with an increased bcl-2 level in gilz KO B lymphocytes. Bcl-2 plays an important role in cancer and can contribute to neoplastic cell expansion by preventing normal cell turnover caused by physiologic cell death mechanisms.41,42 Increased expression of Bcl-2 is found in a wide variety of human cancers, including follicular B-cell lymphoma and B-lymphoblastic leukemia/lymphoma.43 Moreover many patients with persistent polyclonal B-cell lymphocytosis have increased Bcl-2 protein expression.44 Conversely, defects in Bcl-2–regulated apoptosis have been reported to cause or correlate with autoimmunity.45
It has been demonstrated that NF-κB induces Bcl-2 expression in B lymphocytes and inhibits B-cell apoptosis.46,47 We have previously shown that GILZ is able to inhibit transcriptional activity of NF-κB.18,19,37 Inhibition of NF-κB function by GILZ has been also shown in other cell types including macrophages25,34 and epithelial cells.33 Importantly, we have now demonstrated that GILZ inhibits NF-κB also in B cells. Increased levels of nuclear p65/NF-κB in GILZ-deficient cells is consistent with increased occupancy of p65/NF-κB on Bcl-2 promoter, establishing GILZ as an important regulator of NF-κB activity in B cells.
We have also demonstrated that a lack of GILZ impairs GC-induced apoptosis in B lymphocytes. The data presented here clearly show that B cells lacking GILZ are partially resistant to Dex-induced cell death. However, the Dex-induced decrease in the NF-κB presence at the Bcl-2 promoter observed in WT and gilz KO cells was similar. The fact that the Dex-induced decrease of NF-κB binding to Bcl-2 promoter in WT and gilz KO cells is comparable points to the involvement of other mechanisms induced by Dex treatment.48 Altogether, these data suggest that GILZ contributes in part to GC-induced apoptosis, but that the GILZ involvement in GC-induced downregulation of Bcl-2 expression and NF-κB activity in B cells appears to be minor.
Moreover, analysis of Dex-induced apoptosis in distinct B-cell developmental subsets shows that GILZ contributes to GC-induced apoptosis in more differentiated PreB and immature B cells, whereas lack of GILZ does not affect Dex-induced cell death in highly GC-sensitive Pre-ProB and ProB cells. Moreover, GILZ exerts proapoptotic effects in mature B cells, resistant to pharmacologic doses of GC.49,50 Thus, results confirm the role of GILZ in the control of B-cell survival as a mediator of proapoptotic effects of GC, starting from the PreB stage of development.
GC have a great clinical value in the treatment of autoimmune/inflammatory diseases and lymphoid neoplasms, despite an incomplete understanding of their mechanism of action. Aberrant B-cell activity contributes to the development of certain autoimmune/inflammatory pathologies. Therefore, deregulation of GILZ that acts as a regulator of B-cell maintenance could be implicated to disease predisposition. Furthermore, several malignant hematopoietic cells are sensitive to GC-induced apopotosis.51-56 However, long-term GC therapy of leukemia/lymphoma or chronic autoimmune/inflammatory diseases is not recommended because of frequent resistance and relapse, as well as prominent side effects of long-term GC treatment.57-59 GILZ may represent a new potential therapeutic target in the treatment of hematologic malignancies and of autoimmune/inflammatory diseases with fewer adverse side effects than GC. Further studies are warranted to unravel the role of GILZ to predisposition in leukemia/lymphoma progression or B cell–mediated autoimmunity. In this respect, GILZ could be a new diagnostic marker to predict susceptibility and/or resistance in B-cell disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (IG 14291) (C. R.).
Authorship
Contribution: S.B., M.B., D.S., O.B., and C.R. designed the experiments; S.B., M.B., D.S., M.C., T.F., E.M., and P.S. performed the experiments; S.B., M.B., D.S., O.B., E.M., P.S., and C.R. analyzed the data; and S.B., M.B., and C.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo Riccardi, Department of Medicine, Section of Pharmacology, University of Perugia, Piazza Severi 1, 06132 Perugia, Italy; e-mail: carlo.riccardi@unipg.it.
References
Author notes
S.B. ad M.B. contributed equally to this study.