Key Points
CD9 expression enhances the CXCL12-induced migration of pre-B leukemic lymphocytes via RAC1 signaling.
CD9 influences the chemotactic migration and engraftment of pre-B leukemic cells in NOD/SCID mouse testis.
Abstract
CD9, a member of the tetraspanin family, has been implicated in hematopoietic and leukemic stem cell homing. We investigated the role of CD9 in the dissemination of B acute lymphoblastic leukemia (B-ALL) cells, by stably downregulating CD9 in REH and NALM6 cells. CD9 expression was associated with higher levels of REH cell adhesion to fibronectin and C-X-C motif chemokine receptor 4 (CXCR4)-mediated migration. Death occurred later in NOD/SCID mice receiving REH cells depleted of CD9 for transplantation than in mice receiving control cells. After C-X-C motif chemokine ligand 12 (CXCL12) stimulation, CD9 promoted the formation of long cytoplasmic actin-rich protrusions. We demonstrated that CD9 enhanced RAC1 activation, in both REH cells and blasts from patients. Conversely, the overexpression of a competing CD9 C-terminal tail peptide in REH cytoplasm decreased RAC1 activation and cytoplasmic extension formation in response to CXCL12. Finally, the inhibition of RAC1 activation decreased migration in vitro, and the depletion of RAC1 protein from transplanted REH cells increased mouse survival. Furthermore, a testis-conditioned medium induced the migration of REH and NALM6 cells, and this migration was impeded by an anti-CD9 antibody. The level of CD9 expression also influenced the homing of these cells in mouse testes. These findings demonstrate, for the first time, that CD9 plays a key role in the CXCR4-mediated migration and engraftment of B-ALL cells in the bone marrow or testis, through RAC1 activation.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, accounting for 25% of all pediatric cancers and 75% of all cases of childhood malignant blood disease. In most cases (85%), ALL affects the B lineage, giving rise to a heterogeneous group of diseases displaying different genetic abnormalities. Five-year event-free survival rates have improved,1 but about 20% of children suffer from ALL relapse in the bone marrow (BM) or at extramedullary sites, such as the testes or ovaries, these sites being particularly frequently affected in cases of late relapse.2-4 It has been suggested that late relapses originate from a preleukemic clone that persists after treatment.5,6 This clone is present at the extramedullary site, where it may undergo new genetic alterations, generating a “new” leukemic clone.
We have shown that several lymphoblastic leukemias can be distinguished on the basis of their CD9 expression.7,8 CD9 is a protein belonging to the tetraspanin superfamily. Tetraspanins are small proteins with a highly conserved tertiary structure consisting of 4 transmembrane domains.9 They are involved in many biological pathways, due to their ability to associate with a wide variety of other transmembrane proteins, most of which are tissue-specific.10 Together, these proteins form a structural platform, referred to as the tetraspanin web, and play an important role in signaling pathways.10,11 CD9 has been implicated in various physiological processes, such as motility, adhesion, and fertilization.12 It also plays a critical role in several pathological processes, including tumor progression and solid tumor metastasis.13,14 Nevertheless, its modes of action remain unclear and are highly dependent on cell type and molecular partner.10-12,15,16 In hematopoietic cells, CD9 expression depends on the stage of differentiation and the location of the cell.16 In leukemia, CD9 enhances metastatic medullary invasion and decreases the survival of mice receiving an injection of pre-B leukemic cells.17,18 CD9 also promotes the chemotactic migration induced by C-X-C motif chemokine ligand 12 (CXCL12) in vitro and the homing to the BM in vivo of human CD34+ hematopoietic stem and progenitor cells.19 CXCL12 or stromal cell-derived factor-1α is a chemokine from the CXC family. CXCL12 was initially found to affect the growth of pro-B cells,20 but has since been shown to be a potent chemoattractant of lymphocytes.21 Its receptor, C-X-C motif chemokine receptor 4 (CXCR4), is highly expressed on B-cell progenitors and acute leukemia cells and plays a major role in their migration.22,23 The CXCL12/CXCR4 axis is thus crucial in hematopoiesis, supporting the homing and engraftment of normal hematopoietic stem cells (HSCs) and leukemic stem cells (LSCs) in BM.23-25 Disruption of the interaction between CXCR4 and CXCL12 with a selective chemical antagonist of CXCR4 (AMD3100 or plerixafor) promotes the massive and rapid mobilization of HSCs into the peripheral blood of healthy mice and human volunteers.26
The purpose of this study was to investigate the effect of CD9 on the CXCL12-induced motility and engraftment of pre-B leukemic lymphocytes. We identified CD9 as a regulator of pre-B lymphoblast adhesion and a new actor in CXCR4-mediated migration and homing, promoting RAC1 activation in response to CXCL12. We confirmed the activation of the CD9-RAC1 signaling pathway in samples from patients. We demonstrated that CD9 enhanced the dissemination of pre-B leukemic cells to the testis, one of the preferential sites for late relapses of ALL. We therefore hypothesized that CD9, through its effects on migration and homing, may be a key player in late relapses of B acute lymphoblastic leukemia (B-ALL).
Materials and methods
Cell lines and leukemic cells from patients
The REH and NALM6 pre-B acute lymphoblastic leukemic cell lines were cultured in RPMI 1640 (Life Technologies) supplemented with 10% decomplemented fetal calf serum (FCS) and 1% antibiotics.
BM leukemia cells were collected at diagnosis, after informed consent had been obtained, in accordance with the declaration of Helsinki. The protocol was approved by the ethics committee of Rennes Hospital (Rennes, France). Mononuclear cells were isolated from BM by successive centrifugations through lymphocytes separation medium (Eurobio).
Cell transfection
REH and NALM6 cells were infected with lentiviral vectors bearing MISSION pLKO.1 shRNA-puro constructs targeting human CD9 (#TRCN000000296958, #TRCN00000057470) and RAC1 (#TRCN 0000004870; #TRCN 0000004873) or with a nontargeted short hairpin RNA (shRNA) from Sigma-Aldrich. Transduced REH and NALM6 cells were maintained in medium containing 0.5 and 0.25 μg/mL puromycin (Invivogen), respectively.
Flow cytometry analysis
Cells were labeled with anti-CD9–fluorescein isothiocyanate (ALB6; Beckman Coulter), anti-CD9-allophycocyanin (M-L13; Becton Dickinson), anti-CXCR4-allophycocyanin (12G5; Becton Dickinson), anti-CD10-phycoerythrin cyanine 7 tandem (HI10a; Becton Dickinson), and anti-CD19–fluorescein isothiocyanate (J3-119; Beckman Coulter) antibodies. Cell immunofluorescence was measured using a Beckman Coulter FACS FC500 and analyzed with the CXP analysis software package.
Migration assays
We incubated 2 × 106 cells with 1 μg/mL anti-CD9 antibody (MEM-61; Abcam), 1 μg/mL immunoglobulin G (IgG) isotype control (IgG1; Sigma-Aldrich), 20 μM plerixafor (Genzyme), a specific CXCR4 inhibitor, 100 nM CCX771, a specific CXCR7 inhibitor, or 100 nM of the control CCX704 (ChemoCentryx), in the absence of serum, for 30 minutes at 4°C, or with 10 or 25 μM NSC23766 (Santa Cruz Biotechnology), a specific RAC inhibitor, for 3 hours at 37°C. Cells were resuspended in RPMI 1640 supplemented with 1% bovine serum albumin and loaded into the upper chamber of a migration system equipped with 8-μm Transwell microporous polycarbonate membranes (Corning-Costar). We added 0.6 mL of CXCL12 (100 ng/mL; Abcam) or testis tissue-conditioned medium (TCM) to the lower chamber. After 5 hours of incubation at 37°C, the cells that had migrated were subjected to trypan blue exclusion and counted. The migration rate was determined as the percentage of cells from the input that had migrated.
Adhesion assay
We coated 96-well plates with 0.1 μg of superfibronectin diluted in phosphate-buffered saline (Sigma-Aldrich), by incubation overnight at 4°C. We then incubated 5 × 105 cells with 1 μg/mL blocking antibody against CD9 or IgG isotype control, in the absence of serum, for 30 minutes at 4°C. The cells were resuspended in medium supplemented with 5% FCS and allowed to adhere to the fibronectin for 90 minutes at 37°C. Nonadherent cells were removed by washing and adherent cells were quantified by colorimetric analysis with the CellTiter 96 AQ One Solution Cell Proliferation Assay (Promega).
CXCR4 internalization
Cells were stimulated by incubation with CXCL12 (200 ng/mL) for 20 minutes at 37°C and washed in glycine-HCl buffer (50 mM glycine; 150 mM HCl). CXCR4 expression was analyzed by flow cytometry.
Confocal microscopy
Cytospin preparations of cells were fixed in 4% paraformaldehyde for 20 minutes. Preparations were blocked for 1 hour with 5% FCS, supplemented with 0.2% saponin (Sigma-Aldrich) if permeabilization was required. Slides were incubated overnight at 4°C with a mouse anti-CD9 antibody (1:50; ALB6; Beckman Coulter) and a rabbit anti-CXCR4 antibody (1:50; polyclonal; Abcam) or a rabbit anti-CXCR7 antibody (1:500; polyclonal; Abcam). They were then incubated for 1 hour at room temperature with anti-rabbit DyLight594 antibody or anti-mouse DyLight488 secondary antibodies (1:500; Jackson ImmunoResearch Laboratories). Actin filaments were decorated by incubation for 1 hour at room temperature with rhodamine-phalloidin (1:100; Interchim).
Immunoprecipitation assay
Cells were stimulated with CXCL12 (100 ng/mL) for 30 seconds and lysed in 100 μL of lysis buffer (5× Mg2+ lysis/wash buffer [Millipore], 1× protease inhibitor cocktail [Roche], 1 mM sodium orthovanadate, 40 mM β-glycerophosphate, 30 mM sodium fluoride, and 10% glycerol). RAC1-GTP was immunoprecipitated with RAC/CDC42-PAK1 beads (Millipore), used according to the manufacturer’s instructions. Immunoprecipitates were analyzed by western blotting.
Western blot analysis
Cell lysates were separated by electrophoresis in 15% polyacrylamide gels under standard conditions. The separated proteins were blotted onto Hybond-C Extra nitrocellulose membranes (GE Healthcare). Immunoblotting was performed with a 1:1000 dilution of mouse anti-RAC1 or rabbit anti-CXCR4 antibody.
Competition assays with permeant peptides
Permeant peptides labeled at the N terminus with tetramethylrhodamine (TAMRA) and bearing the C-terminal sequence of CD9 or a scrambled sequence were purchased from Lifetein: YGRKKRRQRRR-CCAIRRNREMV (CD9); YGRKKRRQRRR-NRACIRERVCM (scrambled).
Xenograft transplantation and survival analysis
NOD/scid IL2 Rgnull mice (Charles River Laboratories) were maintained in the ARCHE Animal Center (UMS CNRS 3480 Biosit, Rennes, France) at Rennes 1 University. Four-week-old mice received 2 intraperitoneal injections of 20 μg/g busulfan (Busilvex; Pierre Fabre) on 2 days. They were then allowed to rest for 2 days before the IV injection of REH cells (1 × 105, 10 × 106 cells).
Survival analysis was performed on the mice into which 1 × 105 cells were injected. The mice into which 10 × 106 cells were injected were killed 4 weeks after the injection, and single-cell suspensions were prepared from their BM and testes. Pieces of testis tissue were dissociated in 0.25% trypsin (Life Technologies) supplemented with collagenase type 1 (Sigma-Aldrich) for 25 minutes at 37°C. Red blood cells of BM aspirates from mouse femurs were lysed in ammonium-chloride-potassium lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 500 mM EDTA). Human cells were detected by flow cytometry. Dead cells were excluded from the analysis on the basis of their size and granularity characteristics.
These experiments were approved by the ethics committee for animal experimentation of the French Ministry for Higher Education and Scientific Research.
Statistics
Statistical significance was analyzed using nonparametric tests (Wilcoxon or Mann-Whitney), with values of P < .05 considered significant. For survival studies, Kaplan-Meier curves were generated with Prism 6 software and analyzed with Mantel-Cox tests.
Results
CD9 increases the mobility of REH cells in vitro and decreases the survival of mice after the transplantation of REH cells
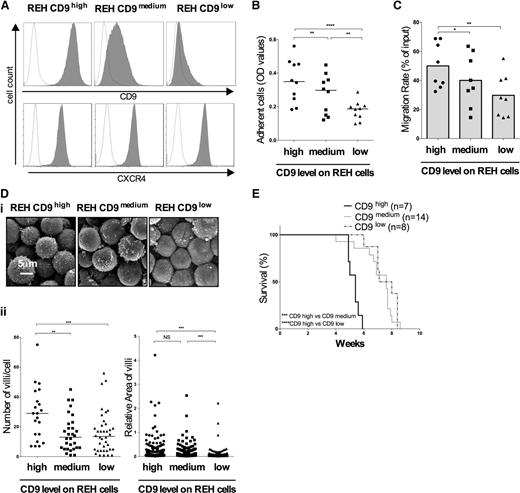
CD9 has been reported to regulate the adhesion of several pre-B cell lines to fibronectin, by modulating β1 integrin avidity.27,28 For confirmation of these observations in the context of B-ALL, we performed adhesion experiments with the REH cell line, a CD9-positive pre-B-ALL cell line established from an ETV6/RUNX1-positive late relapse. We generated 2 stable cell lines depleted of CD9 protein with 2 different shRNAs targeting CD9 (Figure 1A). We found that higher levels of CD9 protein resulted in the adhesion of larger numbers of cells to the fibronectin coat (Figure 1B). We assessed the role of CD9 in the migration of B-ALL cells, using the CXCL12 chemokine as a chemoattractant. REH cells express high levels of CXCR4 and this expression was not modified by transduction with lentiviruses (Figure 1A). Passive migration was initially excluded through the use of CXCL12-depleted medium (average migration rate: 3% ± 1.1%). CD9 enhanced chemotactic migration in vitro (Figure 1C).
Phenotypic and functional effects of CD9 downregulation. REH cells were transduced with 2 different shRNAs targeting CD9 messenger RNA (mRNA). (A) Membrane expression of CD9 and CXCR4 measured by flow cytometry and represented by gray histograms. Fluorescence with the control isotype is indicated by the black line on the histograms. (B) Adhesion assay. REH cells were used to seed superfibronectin-coated 96-well plates. The adherent cells were counted in an MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium) proliferation assay. The graph represents the optic density (OD) values, with the bars indicating the median values. **P < .01, ****P < .0001 in Wilcoxon test. (C) Migration assay. Cell migration in response to a gradient of CXCL12 (100 ng/mL) was measured in a Boyden chamber. Results are presented as migration rates using a scatter dot plot representation. The histograms indicate the means of 8 independent experiments. *P < .05 in Wilcoxon test. (Di) The effects of CD9 depletion on REH cell morphology were observed by scanning electron microscopy. (Dii) The number of villi per cell and the area of the membrane villi were determined with ImageJ software. The bars indicate the median values. n is the number of cells analyzed: REH CD9high, n = 21; REH CD9medium, n = 41; REH CD9low, n = 21; **P < .01, ***P < .001 in Mann-Whitney tests. (E) REH cells stably transduced with shRNA targeting CD9 were cultured and injected IV (105 cells) into 4-week-old NSG mice. The general condition of the mice was monitored daily until their death. Kaplan-Meier survival curves were plotted. n is the number of mice used: REH CD9high, n = 7; REH CD9medium, n = 14; REH CD9low, n = 8;*** P < .001, ****P < .0001 in log-rank (Mantel-Cox) tests.
Phenotypic and functional effects of CD9 downregulation. REH cells were transduced with 2 different shRNAs targeting CD9 messenger RNA (mRNA). (A) Membrane expression of CD9 and CXCR4 measured by flow cytometry and represented by gray histograms. Fluorescence with the control isotype is indicated by the black line on the histograms. (B) Adhesion assay. REH cells were used to seed superfibronectin-coated 96-well plates. The adherent cells were counted in an MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium) proliferation assay. The graph represents the optic density (OD) values, with the bars indicating the median values. **P < .01, ****P < .0001 in Wilcoxon test. (C) Migration assay. Cell migration in response to a gradient of CXCL12 (100 ng/mL) was measured in a Boyden chamber. Results are presented as migration rates using a scatter dot plot representation. The histograms indicate the means of 8 independent experiments. *P < .05 in Wilcoxon test. (Di) The effects of CD9 depletion on REH cell morphology were observed by scanning electron microscopy. (Dii) The number of villi per cell and the area of the membrane villi were determined with ImageJ software. The bars indicate the median values. n is the number of cells analyzed: REH CD9high, n = 21; REH CD9medium, n = 41; REH CD9low, n = 21; **P < .01, ***P < .001 in Mann-Whitney tests. (E) REH cells stably transduced with shRNA targeting CD9 were cultured and injected IV (105 cells) into 4-week-old NSG mice. The general condition of the mice was monitored daily until their death. Kaplan-Meier survival curves were plotted. n is the number of mice used: REH CD9high, n = 7; REH CD9medium, n = 14; REH CD9low, n = 8;*** P < .001, ****P < .0001 in log-rank (Mantel-Cox) tests.
We also used scanning electron microscopy to explore whether CD9 expression induced morphologic changes. CD9 downregulation resulted in a significantly smaller number of villi, which were also significantly thinner on REH CD9low cells than on control cells (Figure 1D).
Finally, we investigated whether the phenotype and mobility properties induced by CD9 downregulation affected engraftment capacity in vivo. We performed xenotransplantation in NSG mice. We first checked that proliferation was not affected by CD9 expression (supplemental Figure 1A, see supplemental Data available at the Blood Web site). We then assessed BM invasion after transplantation, at death. BM invasion rates of 97% were recorded at the time of death, consistent with death from leukemia (supplemental Figure 2). We carried out an analysis of survival curves and demonstrated that the injection of REH cells in which CD9 was downregulated was associated with significantly longer survival (Figure 1E).
CD9 expression influences the CXCL12/CXCR4-mediated migration of B-ALL cells
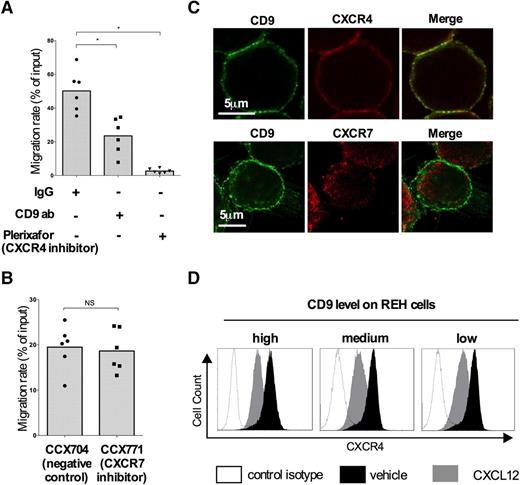
We investigated the contribution of CD9 and CXCR4 to cell migration in response to CXCL12. The inhibition of CXCR4 signaling with plerixafor abolished cell migration. The anti-CD9 antibody decreased migration by almost 47% (average migration rate: 23.51% ± 10.57%), suggesting a possible role of CD9 in this major migration pathway (Figure 2A). We also explored the contribution of a second CXCL12 receptor, CXCR7, the function of which in hematopoietic cell migration remains a matter of debate. We added a specific synthetic inhibitor of CXCR7 (CCX771) to the experimental system described in “Materials and methods.” No effect on REH migration was detected (Figure 2B). We used confocal imaging to localize CD9, CXCR4, and CXCR7 on the cell membrane. Cells were stabilized by cytospin centrifugation at low speed, to conserve their morphology and membrane structures. A merged image of the CD9 and CXCR4 signals showed tight colocalization, confirming that the proteins were located close together on the membrane unlike CD9 and CXCR7 (Figure 2C). The same results have been obtained with NALM6 cells, another pre-B cell line (supplemental Figure 3). We investigated the possible effect on CXCR4 trafficking of the presence of CD9 on the cell membrane, by assessing CXCR4 internalization after stimulation with CXCL12 (Figure 2D). The levels of CXCR4 expression after stimulation were similar in REH CD9high, CD9medium, and CD9low cells, suggesting that CD9 does not affect CXCR4 signaling through enhanced internalization.
CD9 does not affect CXCR4 localization and internalization. Migration assay. Chemotactic migration toward CXCL12 (100 ng/mL) of REH cells after preincubation with 1 μg/mL anti-CD9 antibody and 20 µM plerixafor (A) or CCX771 (100 nM) (B) was measured in a Boyden chamber. The migration rates are represented using a scatter dot plot with the histograms indicating the means of 6 independent experiments. *P < .05 in Wilcoxon test. (C) Immunofluorescence. Cells were subjected to cytospin centrifugation and fixed in 4% paraformaldehyde. The CXCR4, CXCR7, and CD9 proteins were labeled with mouse anti-CD9 (1:50), rabbit anti-CXCR4 (1:50), and rabbit anti-CXCR7 (1:500) antibodies. Confocal imaging of serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil-immersion objective and ×6 zoom. The yellow spots on the merged images indicate the colocalization of CXCR4 and CD9. The images are representative of 3 independent experiments. (D) REH cells were stimulated with CXCL12 (200 ng/mL) for 20 minutes at 37°C. CXCR4 expression on the membrane was assessed by flow cytometry. The histograms show CXCR4 expression in the absence of stimulation (black peaks) and after stimulation (gray peaks), as assessed by flow cytometry. The black lines indicate the fluorescence of REH cells labeled with an isotypic control antibody.
CD9 does not affect CXCR4 localization and internalization. Migration assay. Chemotactic migration toward CXCL12 (100 ng/mL) of REH cells after preincubation with 1 μg/mL anti-CD9 antibody and 20 µM plerixafor (A) or CCX771 (100 nM) (B) was measured in a Boyden chamber. The migration rates are represented using a scatter dot plot with the histograms indicating the means of 6 independent experiments. *P < .05 in Wilcoxon test. (C) Immunofluorescence. Cells were subjected to cytospin centrifugation and fixed in 4% paraformaldehyde. The CXCR4, CXCR7, and CD9 proteins were labeled with mouse anti-CD9 (1:50), rabbit anti-CXCR4 (1:50), and rabbit anti-CXCR7 (1:500) antibodies. Confocal imaging of serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil-immersion objective and ×6 zoom. The yellow spots on the merged images indicate the colocalization of CXCR4 and CD9. The images are representative of 3 independent experiments. (D) REH cells were stimulated with CXCL12 (200 ng/mL) for 20 minutes at 37°C. CXCR4 expression on the membrane was assessed by flow cytometry. The histograms show CXCR4 expression in the absence of stimulation (black peaks) and after stimulation (gray peaks), as assessed by flow cytometry. The black lines indicate the fluorescence of REH cells labeled with an isotypic control antibody.
CD9 promotes the formation of actin protrusions in response to CXCL12
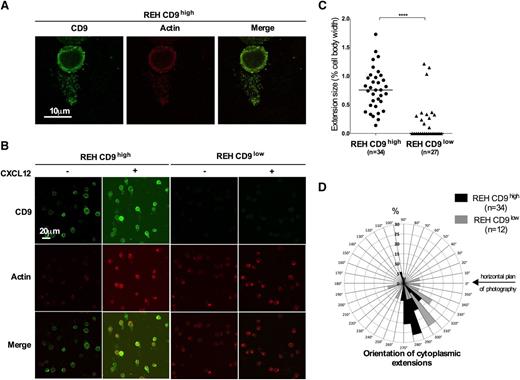
We used confocal microscopy to explore the distributions of CD9 and actin. Following stimulation with CXCL12, REH CD9high cells were found to have long cytoplasmic extensions displaying colocalized CD9 and actin signals (Figure 3A). These structures were numerous on REH CD9high cells, but were clearly less abundant on REH CD9low cells (Figure 3B). The extensions of REH CD9high cells were also significantly longer, increasing the width of the cell by 77% ± 36% rather than by the 21% ± 36% observed for REH CD9low cells (Figure 3C). REH CD9high cell extensions were also essentially unidirectional, with 88% orientated in the same direction (Figure 3D). By contrast, the extensions of REH CD9low cells were more uniformly organized around the cell. In parallel, we measured a significantly larger increase in F-actin content following CXCL12 stimulation in CD9high than in CD9low cells (supplemental Figure 4). These data suggest that CD9 promotes the formation of cytoplasmic extensions and the rearrangement of actin in response to CXCL12.
CD9 promotes the formation of long, actin-rich cytoplasmic protrusions. (A-B) Immunofluorescence. Cells were incubated for 30 seconds at 37°C with 100 ng/mL CXCL12, subjected to cytospin centrifugation, fixed, and permeabilized. Actin filaments were decorated with rhodamine-phalloidin (1:100) and CD9 was labeled with mouse anti-CD9 antibody (1:50). Confocal imaging of serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil objective, zoom x3 (A) and ×1(B). The yellow spots on the merged images correspond to the colocalization of actin and CD9. The images shown are representative of 3 independent experiments. (C) The membrane extensions were measured with ImageJ software. The bars indicate the median values. n is the number of cells analyzed: REH CD9high, n = 34; REH CD9low, n = 27; ****P < .0001 in Mann-Whitney tests. (D) The distribution of membrane extensions is shown as the percentage of extensions oriented in a range of 10°. n indicates the number of cytoplasmic extensions analyzed: REH CD9high, n = 34 (black); REH CD9low, n = 12 (gray).
CD9 promotes the formation of long, actin-rich cytoplasmic protrusions. (A-B) Immunofluorescence. Cells were incubated for 30 seconds at 37°C with 100 ng/mL CXCL12, subjected to cytospin centrifugation, fixed, and permeabilized. Actin filaments were decorated with rhodamine-phalloidin (1:100) and CD9 was labeled with mouse anti-CD9 antibody (1:50). Confocal imaging of serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil objective, zoom x3 (A) and ×1(B). The yellow spots on the merged images correspond to the colocalization of actin and CD9. The images shown are representative of 3 independent experiments. (C) The membrane extensions were measured with ImageJ software. The bars indicate the median values. n is the number of cells analyzed: REH CD9high, n = 34; REH CD9low, n = 27; ****P < .0001 in Mann-Whitney tests. (D) The distribution of membrane extensions is shown as the percentage of extensions oriented in a range of 10°. n indicates the number of cytoplasmic extensions analyzed: REH CD9high, n = 34 (black); REH CD9low, n = 12 (gray).
CD9 affects RAC1 activation, thereby affecting REH migration and engraftment
RAC1 is a Rho-GTPase protein known to cycle between an inactive state (guanosine diphosphate-bound) and an active state (guanosine triphosphate [GTP]-bound). RAC1 is activated by CXCL12 in pre-B cells. This activation enhances the formation of actin-rich lamellipodia in diverse cellular functions, including migration.29
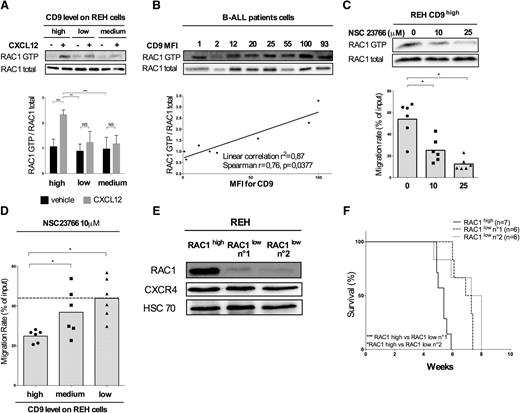
We investigated the effect of CD9 depletion on RAC1 activation. CXCL12 stimulation increased RAC1-GTP protein levels by a factor of >2.5 ± 0.2 in REH CD9high cells (Figure 4A). No such response was observed in REH CD9medium and CD9low cells, suggesting that the modulation of CD9 expression affects RAC1 activation.
CD9 downregulation impairs CXCL12-induced migration by modulating RAC1 activation. (A) REH CD9high, CD9medium, and CD9low cells were stimulated with CXCL12 (100 ng/mL) for 30 seconds at room temperature. RAC1-GTP levels were analyzed by immunoprecipitation followed by western blotting for RAC1. Antibody binding was detected by chemiluminescence (Immobilon kit; Western-Millipore), viewed with the ImageQuant Fuji LAS 4000 Mini (GE Health Biosciences) acquisition system. The intensity of the signals obtained on the western blot was determined with ImageJ software. The graph shows the mean values ± standard deviation (SD) for 3 independent experiments. ***P < .001 in Student t test. (B) RAC1 activation (RAC1-GTP/RAC1 total) was analyzed according to CD9 expression level (MFI for CD9) in blasts extracted from the BM of B-ALL patients collected on diagnosis (n = 8 patient samples; r = 0.79 P = .0279 in Spearman rank correlation test). (C) REH cells were incubated with NSC23766 (10 μM and 25 μM) for 3 hours at 37°C and stimulated with CXCL12 (100 ng/mL) for 30 seconds at room temperature. RAC1 activation was analyzed by immunoprecipitation followed by western blotting. The migration rates are plotted using a scatter dot plot. The histograms represent the means of 6 independent experiments. *P < .05 in Wilcoxon test. The migration of the cells in response to CXCL12 (100 ng/mL) was also assessed in a Boyden chamber. The graph represents the migration rates obtained in each condition using a scatter dot plot, with the histograms indicating the means of 6 independent experiments. *P < .05 in Wilcoxon test. (D) REH CD9high, CD9medium, and CD9low cells were treated with 10 μM NSC23766 before the assay. The migration of cells in response to a gradient of CXCL12 (100 ng/mL) was assessed in a Boyden chamber, on the basis of CD9 expression. Results are expressed as migration rates and represented using a scatter dot plot. The histograms indicate the means of 6 independent experiments. *P < .05 in Wilcoxon test. (E) REH cells were transduced with 2 shRNAs targeting RAC1 mRNA. RAC1 and CXCR4 protein levels were determined by western blotting. (F) Survival analysis. Cells were injected IV (105 cells) into 4-week-old NSG mice. The general condition of the mice was monitored daily until their death. Kaplan-Meier survival curves were plotted. n is the number of mice used: REH RAC1high, n = 7; REH RAC1low no. 1, n = 6; REH RAC1low no. 2, n = 6; *P < .05 ***P < .001 in log-rank (Mantel-Cox) test.
CD9 downregulation impairs CXCL12-induced migration by modulating RAC1 activation. (A) REH CD9high, CD9medium, and CD9low cells were stimulated with CXCL12 (100 ng/mL) for 30 seconds at room temperature. RAC1-GTP levels were analyzed by immunoprecipitation followed by western blotting for RAC1. Antibody binding was detected by chemiluminescence (Immobilon kit; Western-Millipore), viewed with the ImageQuant Fuji LAS 4000 Mini (GE Health Biosciences) acquisition system. The intensity of the signals obtained on the western blot was determined with ImageJ software. The graph shows the mean values ± standard deviation (SD) for 3 independent experiments. ***P < .001 in Student t test. (B) RAC1 activation (RAC1-GTP/RAC1 total) was analyzed according to CD9 expression level (MFI for CD9) in blasts extracted from the BM of B-ALL patients collected on diagnosis (n = 8 patient samples; r = 0.79 P = .0279 in Spearman rank correlation test). (C) REH cells were incubated with NSC23766 (10 μM and 25 μM) for 3 hours at 37°C and stimulated with CXCL12 (100 ng/mL) for 30 seconds at room temperature. RAC1 activation was analyzed by immunoprecipitation followed by western blotting. The migration rates are plotted using a scatter dot plot. The histograms represent the means of 6 independent experiments. *P < .05 in Wilcoxon test. The migration of the cells in response to CXCL12 (100 ng/mL) was also assessed in a Boyden chamber. The graph represents the migration rates obtained in each condition using a scatter dot plot, with the histograms indicating the means of 6 independent experiments. *P < .05 in Wilcoxon test. (D) REH CD9high, CD9medium, and CD9low cells were treated with 10 μM NSC23766 before the assay. The migration of cells in response to a gradient of CXCL12 (100 ng/mL) was assessed in a Boyden chamber, on the basis of CD9 expression. Results are expressed as migration rates and represented using a scatter dot plot. The histograms indicate the means of 6 independent experiments. *P < .05 in Wilcoxon test. (E) REH cells were transduced with 2 shRNAs targeting RAC1 mRNA. RAC1 and CXCR4 protein levels were determined by western blotting. (F) Survival analysis. Cells were injected IV (105 cells) into 4-week-old NSG mice. The general condition of the mice was monitored daily until their death. Kaplan-Meier survival curves were plotted. n is the number of mice used: REH RAC1high, n = 7; REH RAC1low no. 1, n = 6; REH RAC1low no. 2, n = 6; *P < .05 ***P < .001 in log-rank (Mantel-Cox) test.
This result was confirmed in blasts extracted from fresh BM collected from B-ALL patients on diagnosis (Figure 4B). Linear regression analysis revealed a strong correlation between CD9 protein levels (mean fluorescence intensity [MFI] for CD9) and RAC1-GTP:RAC1 total ratio (r2 = 0.87, Spearman r = 0.76 and P = .0377).
We further assessed the effect of RAC1 activation on the chemotactic migration of REH cells. REH CD9high cells were incubated with various concentrations of NSC23766 before the migration assay. NSC23766 prevents RAC1 activation and chemotactic migration in vitro, in a dose-dependent manner (Figure 4C). Moreover, the chemotactic migration of REH CD9high cells was more strongly altered by NSC23766 treatment than that of REH CD9medium and CD9low cells, resulting in higher rates of migration for these cells with low levels of CD9 (Figure 4D). This finding supports the hypothesis that CD9 is involved in RAC1 activation and affects CXCL12-induced migration.
Finally, we explored the impact of RAC1 downregulation on mouse survival. We used 2 different shRNAs to generate 2 cell lines displaying a stable depletion of RAC1 protein (Figure 4E). CXCR4 expression was not affected by transduction or cell proliferation in vitro following RAC1 depletion (supplemental Figure 1B). Finally, xenograft studies in NSG mice showed that survival was significantly longer for mice receiving injections of cells depleted of RAC1 than for mice receiving control cells (Figure 4F). Thus, RAC1 depletion results in phenotypes identical to those observed following CD9 disruption.
The C-terminal tail of CD9 is involved in RAC1 activation
We investigated the mechanism by which CD9 protein affects RAC1 activation, using a permeant TAMRA-coupled peptide containing either the C-terminal amino-acid sequence of CD9 or a scrambled sequence. Cells were incubated with each of these peptides separately, at a concentration of 1 µM. After 2 hours of incubation, the peptides were detectable in the cytoplasm of REH cells and were not trapped in the cytoplasmic membrane (Figure 5A).
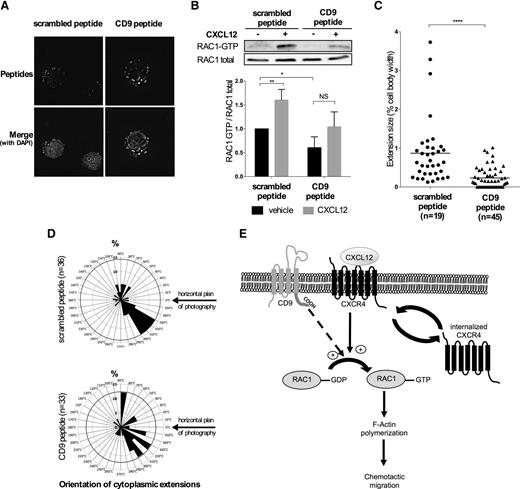
CD9 increases RAC1 activation through its cytoplasmic carboxy-terminal sequence. (A) REH cells were incubated with 1 μM CD9 or scrambled permeant (SCR) peptide for 2 hours. Cells were then subjected to cytospin centrifugation and fixed with 4% paraformaldehyde. The incorporation of fluorescent peptides was monitored with the DeltaVision Epifluorescence Microscope Imaging System. (B-D) REH cells were incubated for 2 hours with 1 μM CD9 or scrambled permeant peptide and were stimulated for 30 seconds with CXCL12 (100 ng/mL). (B) RAC1 activation was analyzed by immunoprecipitation, followed by western blotting for RAC1. The intensity of the signals on the blot was determined with ImageJ software. The graph shows the means ± SD of 3 independent experiments. **P < .01 *P < .05 in Student t test. (C) Cells were subjected to cytospin centrifugation, fixed in 4% paraformaldehyde, and labeled with mouse anti-CD9 antibody (1:50). Confocal imaging on serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil objective. The membrane extensions were measured with ImageJ software. The bars indicate the median values for each set of conditions. n is the number of cells analyzed: SCR peptide, n = 19; CD9 peptide, n = 45 cells; ****P < .0001 in a Mann-Whitney test. (D) The distribution of membrane extensions is represented according to the percentage of extensions oriented in a range of 10°. n is the number of cytoplasmic extensions analyzed: SCR peptide, n = 36, CD9 peptide, n = 33. (E) Model for CD9 regulation of RAC1 activation and chemotactic migration in B-ALL.
CD9 increases RAC1 activation through its cytoplasmic carboxy-terminal sequence. (A) REH cells were incubated with 1 μM CD9 or scrambled permeant (SCR) peptide for 2 hours. Cells were then subjected to cytospin centrifugation and fixed with 4% paraformaldehyde. The incorporation of fluorescent peptides was monitored with the DeltaVision Epifluorescence Microscope Imaging System. (B-D) REH cells were incubated for 2 hours with 1 μM CD9 or scrambled permeant peptide and were stimulated for 30 seconds with CXCL12 (100 ng/mL). (B) RAC1 activation was analyzed by immunoprecipitation, followed by western blotting for RAC1. The intensity of the signals on the blot was determined with ImageJ software. The graph shows the means ± SD of 3 independent experiments. **P < .01 *P < .05 in Student t test. (C) Cells were subjected to cytospin centrifugation, fixed in 4% paraformaldehyde, and labeled with mouse anti-CD9 antibody (1:50). Confocal imaging on serial Z stacks was performed with a Leica SP5 confocal microscope equipped with a 63×/1.4 oil objective. The membrane extensions were measured with ImageJ software. The bars indicate the median values for each set of conditions. n is the number of cells analyzed: SCR peptide, n = 19; CD9 peptide, n = 45 cells; ****P < .0001 in a Mann-Whitney test. (D) The distribution of membrane extensions is represented according to the percentage of extensions oriented in a range of 10°. n is the number of cytoplasmic extensions analyzed: SCR peptide, n = 36, CD9 peptide, n = 33. (E) Model for CD9 regulation of RAC1 activation and chemotactic migration in B-ALL.
We investigated RAC1 activation in the presence of the 2 peptides (Figure 5B). The CD9 peptide significantly decreased basal levels of RAC1-GTP (P = .04) and lower levels of RAC1 activation in response to CXCL12 were reported for this peptide than for the scrambled peptide. Cells expressing the scrambled peptide had long cytoplasmic extensions (Figure 5C), most of which (41.6%) were oriented in the same direction (Figure 5D). By contrast, cells expressing the CD9 peptide had a smaller number of extensions and their extensions were shorter (Figure 5C) and more uniformly distributed around the cell (Figure 5D). These findings suggest that CD9 enhances RAC1 activation and actin remodeling via its C-terminal sequence, in response to CXCL12 (Figure 5E).
CD9 promotes B-cell chemotactic migration in response to TCM, through RAC1 signaling
As the testis is a major site of late relapses and CXCL12 is expressed in mouse testis tissue (supplemental Figure 5), we assessed the biological relevance of this molecule in the migration induced by a TCM. The cells that had migrated were detected by flow cytometry. We determined the migration rate as the percentage of REH cells present in the lower chamber after incubation (Figure 6A).
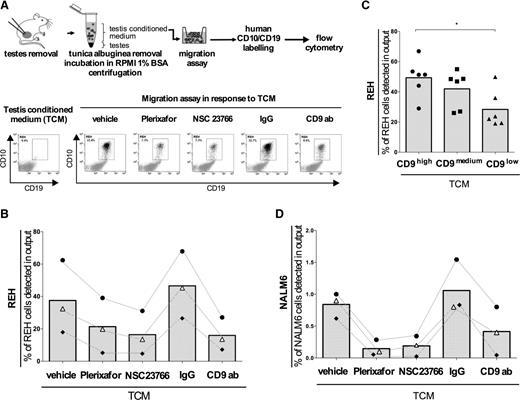
CXCR4 signaling is the main pathway involved in the chemotactic migration of B-ALL cells induced by a TCM. (A) Workflow. After removal of the tunica albuginea, pieces of testis tissue were incubated in RPMI 1640 containing 1% bovine serum albumin for 90 minutes at 37°C. The TCM was collected by centrifugation and still contained mouse testis cells. The bottom left panel illustrates the CD10/CD19-negative status of testis cells still present in TCM obtained by flow cytometry. REH cells that had been incubated with plerixafor (20 μM), NSC23766 (25 μM), anti-CD9 blocking antibody, or IgG isotype control (1 μg/mL), were allowed to migrate in a Boyden chamber in response to TCM. After 5 hours, the cells present in the lower chamber of the migration system were labeled with human anti-CD10 and anti-CD19 antibodies and subjected to flow cytometry. The migration rate was defined as the percentage of human cells detected in the lower chamber after migration. The bottom right panel illustrates the results obtained for a set of experiments with REH cells. (B) The migration rates of 3 independent experiments (•, ♦, ∆) are presented using a scatter dot plot representation with dotted lines connecting the results obtained in the same set of experiments. The histograms indicate the mean for each condition. (C) REH CD9high, CD9medium, and CD9low cells migration in response to TCM was tested. The results are presented using a scatter dot plot where the histograms represent the means of 6 independent experiments. *P < .05 in Wilcoxon test. (D) NALM6 cells migration in response to TCM after a pretreatment with plerixafor, NSC23766, and anti-CD9 antibody was tested. The scatter dot plot represents the results of 3 independent experiments (•, ♦, ∆) and the dotted lines connect the results obtained in the same set of experiments. The histograms indicate the mean for each condition.
CXCR4 signaling is the main pathway involved in the chemotactic migration of B-ALL cells induced by a TCM. (A) Workflow. After removal of the tunica albuginea, pieces of testis tissue were incubated in RPMI 1640 containing 1% bovine serum albumin for 90 minutes at 37°C. The TCM was collected by centrifugation and still contained mouse testis cells. The bottom left panel illustrates the CD10/CD19-negative status of testis cells still present in TCM obtained by flow cytometry. REH cells that had been incubated with plerixafor (20 μM), NSC23766 (25 μM), anti-CD9 blocking antibody, or IgG isotype control (1 μg/mL), were allowed to migrate in a Boyden chamber in response to TCM. After 5 hours, the cells present in the lower chamber of the migration system were labeled with human anti-CD10 and anti-CD19 antibodies and subjected to flow cytometry. The migration rate was defined as the percentage of human cells detected in the lower chamber after migration. The bottom right panel illustrates the results obtained for a set of experiments with REH cells. (B) The migration rates of 3 independent experiments (•, ♦, ∆) are presented using a scatter dot plot representation with dotted lines connecting the results obtained in the same set of experiments. The histograms indicate the mean for each condition. (C) REH CD9high, CD9medium, and CD9low cells migration in response to TCM was tested. The results are presented using a scatter dot plot where the histograms represent the means of 6 independent experiments. *P < .05 in Wilcoxon test. (D) NALM6 cells migration in response to TCM after a pretreatment with plerixafor, NSC23766, and anti-CD9 antibody was tested. The scatter dot plot represents the results of 3 independent experiments (•, ♦, ∆) and the dotted lines connect the results obtained in the same set of experiments. The histograms indicate the mean for each condition.
On 3 independent experiments, the migration induced by the conditioned medium was impaired to similar extents by plerixafor, NSC23766, and the antibody targeting CD9 (Figure 6B). Complete CD9 depletion also significantly decreased migration (Figure 6C). Similar results were obtained for the chemotactic migration induced by CXCL12 (supplemental Figure 6A-B) and the conditioned medium (Figure 6D) with NALM6 cell line, which expresses both CD9 and CXCR4.
CD9 improves engraftment in the testis in vivo
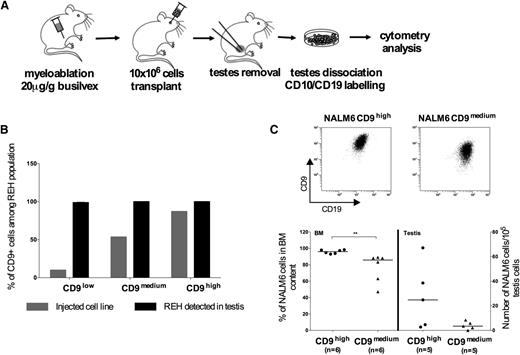
We previously showed that CD9 enhanced the chemotactic migration induced by the CXCL12 present in the extracellular matrix of the testis in vitro. We carried out xenografting studies to assess, in vivo, the effect of CD9 depletion on REH cell engraftment in the testis. Four weeks after injection, testis tissues were collected, dissociated, labeled with human pre-B markers and analyzed by cytometry (Figure 7A). We also assessed CD9 expression. We showed that most of the REH cells able to migrate to testis were positive for CD9, even for the REH CD9low cell line (Figure 7B). This suggests that a CD9-positive subclone within a predominantly CD9-negative population would engraft to the testis tissue more efficiently than the other cells. Similar results have been reported for NALM6 cells displaying stable downregulation of CD9 (CD9medium cells with an MFI for CD9 < 60) (Figure 7C). Three weeks after injection, we observed a small but significant difference in BM homing between NALM6 CD9medium cells and the control cell line (NALM6 CD9high with MFI>100), with the control cells displaying slightly higher rates of BM homing. This effect was particularly marked in the testis, which contained more NALM6 CD9high cells than NALM6 CD9medium cells after xenografting (number of NALM6 cells detected in the testis per 105 testis cells: median value of 24.8 for NALM6 CD9high cells and of 3.43 for NALM6 CD9medium cells).
CD9 expression conditions homing in the testis in vivo. (A) Workflow. REH or NALM6 cells were injected into 4-week-old NSG mice. The mice were killed 4 weeks after xenografting and their testes were collected. After removal of the tunica albuginea, pieces of the testes were dissociated by incubation in 0.25% trypsin and 1 µg/mL collagenase for 30 minutes at 37°C. The cell suspension was filtered through a nylon mesh, labeled with antibodies against CD19, CD10, and CD9, and analyzed by flow cytometry. (B) REH CD9high, CD9medium, and CD9low cells were transplanted into NSG mice. The graph shows the percentage of CD9+ cells among REH cells initially injected and REH population found in the testis after xenografting. n is the number of mice undergoing transplantation: REH CD9low, n = 3; REH CD9medium, n = 4; REH CD9high, n = 2. (C) NALM6 cells in which CD9 was stably downregulated or control cells were injected into NSG mice. Three weeks after injection, the contents of the BM and testes were analyzed. The graph shows the results obtained for 6 mice for each set of conditions. **P < .01 in a Mann-Whitney test.
CD9 expression conditions homing in the testis in vivo. (A) Workflow. REH or NALM6 cells were injected into 4-week-old NSG mice. The mice were killed 4 weeks after xenografting and their testes were collected. After removal of the tunica albuginea, pieces of the testes were dissociated by incubation in 0.25% trypsin and 1 µg/mL collagenase for 30 minutes at 37°C. The cell suspension was filtered through a nylon mesh, labeled with antibodies against CD19, CD10, and CD9, and analyzed by flow cytometry. (B) REH CD9high, CD9medium, and CD9low cells were transplanted into NSG mice. The graph shows the percentage of CD9+ cells among REH cells initially injected and REH population found in the testis after xenografting. n is the number of mice undergoing transplantation: REH CD9low, n = 3; REH CD9medium, n = 4; REH CD9high, n = 2. (C) NALM6 cells in which CD9 was stably downregulated or control cells were injected into NSG mice. Three weeks after injection, the contents of the BM and testes were analyzed. The graph shows the results obtained for 6 mice for each set of conditions. **P < .01 in a Mann-Whitney test.
Discussion
CD9 is one of the best-characterized tetraspanins, but the functional involvement of CD9 in the mobility of leukemic blasts has never before been reported. We found that CD9 upregulated the adhesion of B leukemic cells to fibronectin and the migration of these cells in response to CXCL12, through a pathway dependent on RAC1.
Pre-B cells express a limited range of chemokine receptors, and CXCR4 is one of the most strongly expressed. The CXCL12/CXCR4 axis is of major importance in several biological processes. Its primary role is maintenance of the hematopoietic niche, through the surveillance of signaling and self-renewal and by allowing HSC homing to occur.25 This axis is also essential for the retention of ALL cells in the BM30 and it plays a role in the dissemination of leukemic cells.31-33 We showed, in xenografting experiments on NSG mice, that reduced CD9 expression increased mouse survival, consistent with lower levels of dissemination and engraftment abilities. Our results are consistent with those of a recent study showing that CD9-positive cells are more tumorigenic than their CD9-negative counterparts.17
CD9 and CXCR4 were colocalized on the REH cell membrane. We therefore investigated the effect of CD9 on CXCR4 internalization following CXCL12 binding. CD9 has already been shown to attenuate epidermal growth factor receptor signaling by increasing receptor internalization following epidermal growth factor stimulation.34 However, we found that CD9 depletion had no effect on CXCR4 internalization. By contrast, following CXCL12 stimulation, most of the CD9-positive cells had long cytoplasmic extensions, mostly oriented in the same direction and containing actin filaments not detected in the cells depleted of CD9. Such structures have been observed in B cells stimulated with interleukin-4 and lipopolysaccharide.35 Actin extensions are finger-like protrusions containing parallel bundles of actin filaments that protrude from the leading edge of many motile cells.36 We suggest that CD9 may be a partner in the CXCL12/CXCR4 axis, optimizing the formation of efficient motility structures. Severinson and Westerberg reported that actin filament assembly and growth in B cells was driven by cytokine receptor activation, leading to the production of PIP2 and active Rho-family GTPases.35 Rho--family proteins are molecular switches that alternate between inactivated and activated states. Activation leads to the formation of new actin filaments and their stabilization.36 RAC1 is one of the best studied member of the Rho family; it plays a critical role in actin remodeling during adhesion and migration events.29 Some studies have strongly suggested that RAC1 is a key player in HSC homing and retention in the BM.29,37,38 In leukemia, RAC1 is overexpressed in samples from ALL patients39 and enhances CXCL12-induced migration and homing to BM niches.40 Furthermore, Mangolini et al showed that ETV6/RUNX1 translocation enhances RAC1 signaling which promotes leukemogenesis.41 We show here that CD9 enhances the CXCL12-induced activation of RAC1 via its C-terminal tail. Moreover, as for CD9 depletion, changes in RAC1 activation impaired actin rearrangement, chemotactic migration in vitro, and BM homing in vivo. These results are consistent with the findings of Wang et al, who showed that the CD9 C-terminal tail was involved in the formation of microvilli and filopodia, in which CD9 was expressed and colocalized with F-actin.42 The mechanism by which CD9 enhances RAC1 activation in response to CXCL12 signaling remains unknown, but our findings highlight the mechanisms underlying the motility of leukemic blasts, potentially accounting for chemotactic dissemination of B-ALL in vivo. Indeed, the CXCL12/CXCR4 pathway plays a critical role in cancer, by enhancing the tropism of cancer cells for organs expressing CXCL12.43 Furthermore, in childhood ALL, CXCR4 expression on the cell surface is predictive for extramedullary infiltration.44 CXCL12 is expressed in the testis,45 one of the principal extramedullary sites of late ALL relapses. We demonstrate here that CXCR4 signaling plays a major role in the migration of pre-B lymphoblasts to the testis, as the chemotactic migration induced by a TCM was abolished by plerixafor. CD9 and RAC1 are also key players in this migration. In vivo, the engraftment of pre-B lymphoblasts in the testis is highly dependent on the level of CD9 expression. These results are consistent with those of recent studies on spermatogonial stem cells (SSCs) showing that (1) CXCL12 is a chemotactic factor inducing SSCs to migrate to testis niches,45 (2) CD9 is a marker of the male germline, with testis-repopulating potential,46 and (3) RAC1 enhances transendothelial migration across the blood-testis barrier.47
In summary (Figure 5E), we show here, for the first time, that the tetraspanin CD9 can interfere with CXCL12/CXCR4 signaling by enhancing RAC1 activation in a pre-B ALL cell line and, more importantly, in samples from patients. RAC1 activation enhances actin rearrangement and the formation of cytoplasmic extensions, promoting chemotactic migration toward CXCL12 in the testis, a recognized site of late ALL relapses. These data indicate that CD9 increases the ability of pre-B leukemic cells to disseminate, through its effects on migration and homing, suggesting a possible key role in late relapses of B-ALL, which are currently poorly misunderstood.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Gene Expression and Oncogenesis team for helpful discussions and for providing scientific expertise, Prof Vincent Praloran and Dr Arnaud Villacrecès for the NALM6 cell line and the xenograft protocol for NOD/SCID mice, and Dr Elisabetta Dondi for providing technical expertise on flow cytometry. We thank the Animalerie Rennaise Centre d'Hébergement et d'Expérimentation (Stéphanie Tremulot, Gaëlle Bourgine), flow cytometry and cell sorting (Laurent Deleurme, Gersende Lacombe), and Microscopy Rennes imaging center (Agnès Burel, Marie-Thérèse Lavault) technological platforms from UMS CNRS 3480/US INSERM 018 and Centre de Microscopie Electronique à Balayage et de Microanalyse (Joseph Le Lannic) for technical support and scientific expertise.
This work was supported by la Ligue Régionale contre le Cancer (committees 22, 35, and 56; M.-P.A., V.G., M.-B.T.), European Union FP7 (REA grant no. 291851; M.-B.T.), Association Laurette Fugain (V.G., M.-P.A.), SFR Biosit UMS 3480 (M.-B.T., M.-P.A.), la Région Bretagne (M.-P.A.), l’Institut de la mère et l’enfant de Rennes (M.-P.A.), the CNRS, and Université de Rennes 1.
Authorship
Contribution: M.-P.A. and M.-B.T. designed the study, performed experiments, analyzed the data, and wrote the paper; A.V. designed the study, performed experiments, and analyzed the data; G.R., J.B., and A.-G.R. performed experiments and analyzed the data; N.V.-B. participated in drafting and revising the article; C.L. contributed to the analysis and interpretation of data; M.-D.G. designed the study and wrote the paper; V.G. designed the study, analyzed the data, and wrote the paper; and all authors reviewed the paper and agree with its content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginie Gandemer, IGDR-CNRS UMR 6290, Faculté de Médecine, 2 avenue du Professeur Léon Bernard, CS 34317, 35043 Rennes Cedex, France; e-mail: virginie.gandemer@chu-rennes.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal