To the editor:
Despite its undeniable importance, standard cytogenetic analysis is still not a routine practice for most reference centers in developing countries, and its accomplishment is practically limited to university research centers. Particularly in Brazil, little is known about the cytogenetic and molecular characterization of patients with acute myeloid leukemia (AML), and results about clinical outcomes remain scarce. In an effort to minimize the lack of results and contribute new insights in our setting, we reported clinical features and outcomes of 241 nonselected Brazilian patients with AML (nonacute promyelocytic leukemia) from 2 university hospitals, followed from November 2005 to April 2015.
Patients were classified according morphologic, immunophenotypic, and cytogenetic findings. Treatment protocols were similar between centers. For patients up to 60 years of age, the treatment protocol was adapted according to performance status and the presence of comorbidities (in particular, cardiac disorders). Briefly, the conventional chemotherapy consisted of daunorubicin (45-90 mg/m2 per day for 3 days) and cytarabine (100-200 mg/m2 per day for 7 days) or thioguanine, cytosine arabinoside, and daunorubicin as induction,1 followed by 3 or 4 cycles of consolidation therapy with high doses of cytarabine (>1 g/m2 per day). For patients who did not achieve complete remission (CR) after 1 course of chemotherapy, a second course was administered between days 28 and 35 after the end of the first course. CR was assessed by bone marrow examination on day 28 after each course of chemotherapy. For those who needed it, a postremission therapy based on autologous or allogeneic transplantation was performed. Patients older than 60 years were treated with low-dose cytarabine; a combination of etoposide, thioguanine, and idarubicin; or best supportive care. The local research ethics board of each participating center approved the study. Research was conducted in accordance with the Declaration of Helsinki.
The baseline characteristics are summarized in supplemental Table 1. One hundred thirty patients were enrolled in Recife (northeast Brazil, 54%), and 111 patients were enrolled in Campinas (southeast Brazil, 46%). Baseline features were similar between centers. The median age was 47 years (range, 18-97 years) with 114 males (47%). Sixty-two patients (26%) were older than 60 years. Pretreatment bone marrow samples were analyzed by G-banding cytogenetics, of which 187 (78%) were successful. According to Medical Research Council trials,2 patients were stratified as follows: favorable (30/187, 16%), intermediate (119/187, 64%), and adverse (38/187, 20%). Overall, 101 patients (42%) were cytogenetically normal. To test whether the samples without cytogenetics results were missing at random, the overall survival (OS) was evaluated for patients with and without cytogenetics data. The 5-year OS rate did not differ between patients with (18%) and without (23%) available cytogenetics data (P = .372). Additionally, the entire cohort was fully characterized for NPM1 and FLT3-ITD mutations. Details can be found in the supplemental Data, available on the Blood Web site.
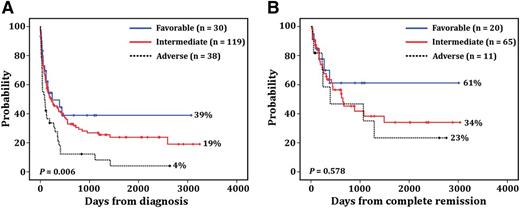
Out of 241 enrolled patients, 39 patients (16%) who started the induction treatment were lost to follow-up without assessment for CR. Of 202 evaluable patients, 115 (57%) achieved complete hematologic remission. CR rates according to the cytogenetic risk stratification were 33%, 64%, and 77% for adverse, intermediate, and favorable groups, respectively (P = .001). The logistic regression analysis revealed that age (odds ratio [OR], 0.95; 95% confidence interval [CI], 0.93-0.98; P = .002) and cytogenetic risk stratification (OR, 0.41; 95% CI, 0.19-0.89; P = .024) were independently associated with CR. The median follow-up among survivors was 177 days (95% CI, 85-268 days). According to Medical Research Council criteria, patients assigned to the adverse group exhibited significantly shorter survival (85 days; 95% CI, 43-169 days) than patients assigned to the intermediate (224 days; 95% CI, 72-375 days) and favorable groups (241 days; 95% CI, 168-572 days) (P = .006; Figure 1A). Age (hazard ratio [HR], 1.1; 95% CI, 1.01-1.2; P = .029), cytogenetic risk stratification (HR, 1.45; 95% CI, 1.02-2.06; P = .036), and white blood cell counts (HR, 1.03; 95% CI, 1-1.06; P = .021) were independently associated with poor OS. The median disease-free survival (DFS) for adverse, intermediate, and favorable groups was 392 days (95% CI, 37-1544 days), 650 days (95% CI, 208-1091 days), and 668 days (95% CI, 57-1278 days), respectively. In contrast to OS, cytogenetic risk stratification had no impact on DFS (P = .578; Figure 1B).
Patient survival. Probability of OS (A) and DFS (B) in patients with AML according to cytogenetic risk stratification.1 The percentages of each group are presented on the graph. The “n” indicates the number of patients included in each analysis.
Patient survival. Probability of OS (A) and DFS (B) in patients with AML according to cytogenetic risk stratification.1 The percentages of each group are presented on the graph. The “n” indicates the number of patients included in each analysis.
In summary, we identified patients with favorable, intermediate, and adverse outcomes with frequencies very similar to those reported by other groups.2,3 However, our patients’ clinical outcomes were significantly inferior to those reported by developed countries. The most significant difference was observed in the favorable group, whose survival rate was significantly lower (39%) than rates reported by studies conducted in the United States and Europe.2,4 Furthermore, the early mortality rate was especially higher in our cohort (42%), which is probably a major cause of lower OS rate. Among the main reasons for high early mortality in Brazil, we highlight the lack of adequate hospital infrastructure, especially during induction therapy.5,6 As a consequence, high incidence of bacterial and fungal infections are frequently reported.7 It is important to note that our results are representative of a real-life setting, which strongly differs from the well-controlled clinical trials conducted in developing countries.
Altogether, our results and others1,5,6,8 draw attention to an urgent need for improved clinical support and treatment of patients with AML in order to obtain results comparable to those reported in developed countries. Such improvements may be achieved through international collaborative efforts, which have already proved their effectiveness in economically less privileged countries. A great example is the International Consortium on Acute Promyelocytic Leukemia study.9 This initiative considerably reduced the differences in treatment outcome of patients with acute promyelocytic leukemia between developed and developing countries through the dissemination of knowledge and exchange of experience from well-established cooperative groups in the United States and Europe. We hope that with better hospital infrastructure and such initiatives, the clinical outcomes of our patients will improve in the near future.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) (2008/57895-1), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (481904/2010-7), and Instituto Nacional de Ciência e Tecnologia do Sangue (565036/2010-6). M.R.d.M. received a fellowship from FAPESP (2007/54686-0).
Contribution: A.S.L. and M.R.d.M. performed experiments, analyzed and interpreted data, and drafted the manuscript; E.F., M.F.B., M.M.O., B.K.D., R.A.d.A., F.R.S., C.F.R., and C.G.M. performed research, collected data, updated the clinical data, and reviewed the manuscript; A.R.L.-A. interpreted and analyzed data, performed statistical analyses, and drafted the manuscript; and K.P., I.L.-M., and M.A.B. conceived and designed the study, provided the samples, reviewed the manuscript, and gave the final approval of the version to be submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio R. Lucena-Araujo, Department of Genetics, Center of Biological Sciences, Federal University of Pernambuco, Av Prof Moraes Rego, 1235, Recife, PE 50670-901, Brazil; e-mail: araujoarl@hotmail.com.
References
Author notes
A.S.L. and M.R.d.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal