To the editor:
Severe congenital neutropenia (CN) is a heterogeneous disease characterized by an absolute neutrophil count (ANC) below 500 cells per microliter and recurrent, life-threatening bacterial infections. Treatment with recombinant human granulocyte colony-stimulating factor (rhG-CSF) was shown to increase ANCs in the majority of patients and dramatically improves the quality of life.1 In a previous study, we demonstrated major differences in responses to G-CSF and granulocyte macrophage CSF (GM-CSF) in patients with CN.2 In the majority of these patients, GM-CSF failed to induce neutrophil granulocyte counts but did induce monocytosis and eosinophilia.2 Many underlying genetic defects have been identified. Among them are mutations in the ELANE, HAX1, and G6PC3 genes and many others.3 A recent study identified patients with biallelic loss-of-function CSF3R mutations who were considered to have a novel subtype of CN.4 These patients did not respond to G-CSF therapy, and no treatment options were available for them. Heterozygous acquired mutations in the CSF3R gene were also reported in CN patients.5
In this study, we identified a CN patient who did not respond to G-CSF treatment. Three days after birth, the female patient was diagnosed with CN with blood ANC below 250 cells per microliter. White blood cell differential counts were as follows: 6% neutrophils, 7% monocytes, 4% eosinophils, and 83% lymphocytes. A bone marrow (BM) evaluation at 3 weeks of age revealed granulopoietic hypoplasia with reduction of all stages but no maturation arrest or increase in blasts. Erythropoiesis, lymphopoiesis, and megakaryopoiesis were normal. No antineutrophil antibodies were detected. Mutational screening revealed no mutations in ELANE, HAX1, or G6PC3. Cytogenetic evaluation revealed a normal karyotype.
Sequencing of the CSF3R gene showed 2 heterozygous mutations in this patient that revealed a compound heterozygous mode of inheritance of CSF3R mutations. In one allele intronic mutation, c.998-2A>T leads to the skipping of exon 9 and introduces an aberrant sequence downstream of exon 8 and a shift in the reading frame. In the second allele, we detected a stop-codon (p.W547*) mutation in the extracellular part of the G-CSF receptor (G-CSFR) (Figure 1A). The p.W547* mutation was inherited from the father and the c.998-2A>T mutation was inherited from the mother.
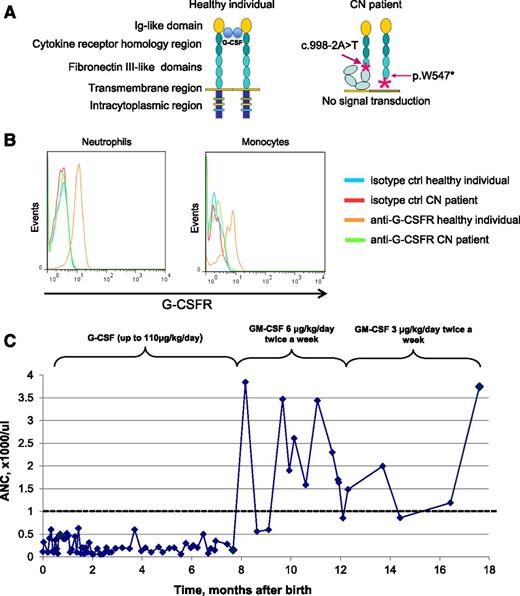
Biallelic CSF3R mutations detected in the CN patient leading to the absence of G-CSFR surface expression and G-CSF unresponsiveness. (A) Schematic representation of the wild-type G-CSFR and the mutant G-CSFR in the CN patient. The wild-type G-CSFR is composed of an extracellular part, a transmembrane region, and an intracellular domain. The extracellular part of the receptor includes an immunoglobulin (Ig)-like module, the cytokine receptor homology domain, and 3 fibronectin type III modules. Upon G-CSF binding to its receptor, a 2:2 tetrameric complex is formed. The intracellular domain is essential for the transduction of proliferation and differentiation signals. Both mutations identified in the CN patient alter the G-CSFR molecular composition either by creating a premature stop-codon or by shifting the codon frame and introducing spurious amino acids. (B) Absence of G-CSFR expression on the surface of the patient’s granulocytes and monocytes. The patient and healthy donor blood samples were stained with an allophycocyanin (APC)-conjugated anti-G-CSFR antibody, and surface G-CSFR expression on the patient’s granulocytes and monocytes was measured by flow cytometry. No surface expression of G-CSFR protein was detected. Cells from healthy donors were used as a positive control (ctrl). Representative histograms showing G-CSFR staining in the CN patient and healthy donor are depicted. (C) ANC and response to GM-CSF/G-CSF in the CN patient during the course of treatment. After 7 months of G-CSF administration, the patient still failed to produce neutrophils in an amount sufficient to protect against recurrent bacterial infection. After GM-CSF administration, the patient’s neutrophil count rose significantly to more than 1000 cells per microliter (range, 860-3744 cells per microliter), allowing the GM-CSF dose to be reduced to 3 µg/kg twice a week. After 6 months of successful treatment with GM-CSF, the patient was again prescribed G-CSF. Because G-CSF therapy produced no effect on neutrophil count, it was replaced with GM-CSF therapy (data not shown).
Biallelic CSF3R mutations detected in the CN patient leading to the absence of G-CSFR surface expression and G-CSF unresponsiveness. (A) Schematic representation of the wild-type G-CSFR and the mutant G-CSFR in the CN patient. The wild-type G-CSFR is composed of an extracellular part, a transmembrane region, and an intracellular domain. The extracellular part of the receptor includes an immunoglobulin (Ig)-like module, the cytokine receptor homology domain, and 3 fibronectin type III modules. Upon G-CSF binding to its receptor, a 2:2 tetrameric complex is formed. The intracellular domain is essential for the transduction of proliferation and differentiation signals. Both mutations identified in the CN patient alter the G-CSFR molecular composition either by creating a premature stop-codon or by shifting the codon frame and introducing spurious amino acids. (B) Absence of G-CSFR expression on the surface of the patient’s granulocytes and monocytes. The patient and healthy donor blood samples were stained with an allophycocyanin (APC)-conjugated anti-G-CSFR antibody, and surface G-CSFR expression on the patient’s granulocytes and monocytes was measured by flow cytometry. No surface expression of G-CSFR protein was detected. Cells from healthy donors were used as a positive control (ctrl). Representative histograms showing G-CSFR staining in the CN patient and healthy donor are depicted. (C) ANC and response to GM-CSF/G-CSF in the CN patient during the course of treatment. After 7 months of G-CSF administration, the patient still failed to produce neutrophils in an amount sufficient to protect against recurrent bacterial infection. After GM-CSF administration, the patient’s neutrophil count rose significantly to more than 1000 cells per microliter (range, 860-3744 cells per microliter), allowing the GM-CSF dose to be reduced to 3 µg/kg twice a week. After 6 months of successful treatment with GM-CSF, the patient was again prescribed G-CSF. Because G-CSF therapy produced no effect on neutrophil count, it was replaced with GM-CSF therapy (data not shown).
No expression of G-CSFR was detected on the patient’s neutrophils or monocytes in contrast to blood cells from the healthy donors (Figure 1B). Granulocyte macrophage CSFR (GM-CSFR) expression on CD33+ cells from the patient’s BM was similar to that observed for BM cells from healthy donors. In addition, we plated the patient’s BM mononuclear cells in a semisolid medium supplemented with 10 ng/mL G-CSF, 10 ng/mL GM-CSF, or a cytokine cocktail containing G-CSF, GM-CSF, interleukin-3, stem cell factor, and erythropoietin, and cultured them for 14 days. No granulocyte colony-forming unit (CFU-G), granulocyte macrophage CFU, or macrophage CFU colonies were found in plates supplemented with 10 ng/mL rhG-CSF. In sharp contrast, all types of colonies grew on plates containing either rhGM-CSF or the cytokine cocktail.
G-CSF treatment of this patient was initiated at the age of 3 weeks, but ANCs failed to increase with doses up to 110 µg/kg per day. At the age of 7 months, treatment with GM-CSF (6 μg/kg per day) was initiated. In the first year of GM-CSF treatment, peripheral blood ANCs ranged from 860 to 3744 cells per microliter, enabling the GM-CSF dose to be reduced to 3 µg/kg per day twice a week (Figure 1C). To evaluate whether GM-CSF was still required to maintain sufficient ANCs, GM-CSF administration was stopped with prophylactic administration of oral antibiotics. Because of the development of otitis media, GM-CSF treatment was restarted at a dose of 3 µg/kg twice a week. This dose was sufficient to maintain the patient’s ANC above 1000 cells per microliter and kept her free from infections. The patient has remained on GM-CSF treatment for the last 12 years without any adverse events.
In summary, we provide the first demonstration of the successful treatment of a CN patient harboring biallelic loss-of-function CSF3R mutations who did not respond to G-CSF by administering GM-CSF. These findings suggest that all CN patients who do not respond to G-CSF should be screened for germ-line CSF3R mutations, and treatment with GM-CSF should be considered. We propose that our patient is a member of a novel genetic subtype of CN, termed CN-CSF3R, which has also been suggested by Triot et al.4
Authorship
Acknowledgments: The authors thank the physicians of the Severe Chronic Neutropenia International Registry for providing patient material and the study patients for their cooperation. This work was supported by grant no. DFG-SK-92/4 from the Madeleine-Schickedanz Kinderkrebsstiftung, the REBIRTH Cluster of Excellence of the Hannover Medical School, the ERA Network of rare diseases, and the Federal Ministry of Education and Research (German Network on Congenital Bone Marrow Failure Syndromes). M.U. was supported by a grant from the North German Supercomputing Alliance (HLRN) and the Madeleine-Schickedanz Kinderkrebsstiftung.
Contribution: M.K., O.K., and A.Z. performed the main experiments; M.U. and S.K. analyzed deep DNA sequencing data for CSF3R; M.K., J.S., and K.W. analyzed the data and wrote the manuscript; S.M.-H. and C.Z. provided patient material and analyzed clinical data; and J.S., K.W., and C.Z. designed and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl Welte, Department of Pediatrics, University Hospital Tuebingen, Hoppe-Seyler-Strasse 1, 72076 Tübingen, Germany; e-mail: karl.welte@med.uni-tuebingen.de
References
Author notes
C.Z. and K.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal