In this issue of Blood, complementary studies by Amin et al and Linley et al demonstrate that sugar moieties linked to surface immunoglobulin (sIg) of follicular lymphoma (FL) cells directly interact with endogenous lectins within the lymphoma niche and lead to activation of downstream B-cell receptor (BCR) signaling pathways. In addition to providing further insight into the role of the microenvironment in lymphomagenesis, these findings expose a unique molecular interaction that may represent a viable target for therapeutic intervention.1,2
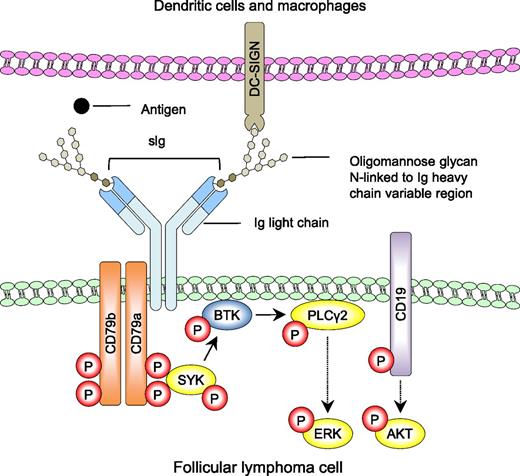
Simplified model of lectin-mediated BCR signaling in follicular lymphoma. N-linked oligomannose glycans within the variable region of sIg prevent binding of cognate antigen (●) but interact directly with mannose-binding lectins (DC-SIGN) expressed by dendritic cells and macrophages within the lymphoid microenvironment. Amin et al and Linley et al demonstrate that these interactions result in organization of the BCR (sIg, CD79a, and Cd79b) and CD19 coreceptor into signaling platforms with subsequent downstream phosphorylation of SYK, PLCγ2, ERK, and AKT (yellow). These signaling pathways can be blocked with inhibitors of SYK and BTK. Red P denotes phosphorylation events. Solid arrows indicate direct interactions and dotted arrows indicate that there are additional intermediates not shown.
Simplified model of lectin-mediated BCR signaling in follicular lymphoma. N-linked oligomannose glycans within the variable region of sIg prevent binding of cognate antigen (●) but interact directly with mannose-binding lectins (DC-SIGN) expressed by dendritic cells and macrophages within the lymphoid microenvironment. Amin et al and Linley et al demonstrate that these interactions result in organization of the BCR (sIg, CD79a, and Cd79b) and CD19 coreceptor into signaling platforms with subsequent downstream phosphorylation of SYK, PLCγ2, ERK, and AKT (yellow). These signaling pathways can be blocked with inhibitors of SYK and BTK. Red P denotes phosphorylation events. Solid arrows indicate direct interactions and dotted arrows indicate that there are additional intermediates not shown.
Despite loss of 1 Ig allele through chromosome translocation with BCL2, FL cells maintain expression of sIg, highlighting the notion that BCR signaling is important for FL cell survival and proliferation. In normal germinal center B cells, the source of this signaling comes from binding of the sIg variable domain to its cognate antigen. However, in FL, the identification of persistent antigens that might activate BCR signaling has been limited to a subset of ∼20% to 25% of cases that express sIg capable of binding autoantigens.3,4 Alternatively, presence of an antigen-independent mechanism for promoting BCR activation is suggested by reports demonstrating lectin binding to FL sIg through N-linked oligomannose glycans present within the antigen-binding domain.5-7
Under normal conditions, somatic hypermutation of the variable region of the Ig genes results in selection of a B cell that produces an antibody with high affinity for its cognate antigen, and N-linked glycosylation is generally restricted to sites within the Ig constant region. In contrast, >80% of FL cases acquire somatic mutations that introduce Asn-X-Ser/Thr motifs into the Ig variable region and function as acceptor sites for N-linked glycosylation.8-10 Resulting glycans added during processing in the endoplasmic reticulum are atypical in that they terminate in mannose sugar moieties, indicating that they do not fully mature through the Golgi complex.5 As a malignancy of germinal center B-cell origin, FL cells demonstrate ongoing somatic hypermutation with clonal heterogeneity. However, acquired glycosylation motifs are conserved, implying that they arise early in lymphomagenesis and impart a selective advantage. Moreover, although antigen recognition is impaired,7 the recurrent localization of mannosylated glycans to the antigen-binding domain suggests an ability to interact with mannose-binding lectins in the microenvironment and possibly influence FL pathophysiology.
Dendritic cell–specific intercellular adhesion molecule-3–grabbing nonintegrin (DC-SIGN) is a lectin that is expressed by dendritic cells and macrophages in normal lymphoid tissue and binds FL cells in vitro through mannosylated glycans on sIg.6 Examination of the FL microenvironment by Linley et al revealed scattered DC-SIGN+ mononuclear cells and a background of FL cells within DC-SIGN+ lymphoid sinuses. Amin et al also demonstrated direct contact of DC-SIGN+ cells with FL cells within paracortical lymphoid sinuses and perifollicular areas and found this lectin to be overexpressed on dendritic cells and tumor-associated macrophages from FL samples compared with reactive tonsils. This suggests that elements of the microenvironment may be organized to favor interactions with FL cells. The additional finding of DC-SIGN expression by FL lymphatic endothelial cells also hints at a potential mechanism for disease dissemination.
Both groups demonstrated heterogeneous binding of recombinant DC-SIGN to sIg on immunoglobulin M–positive (IgM+) FL cells, with Amin et al noting DC-SIGN staining to be significantly higher on IgM+ than IgG+ FL samples. Linley et al suggest that there may be a positive correlation between sIgM expression and DC-SIGN binding, whereas a biochemical analysis by Amin et al also indicates that DC-SIGN high-binding IgM+ FL might have a higher mannose glycosylation profile compared with DC-SIGN low-binding IgM+ FL. Other mannose-binding lectins including endogenous mannose receptor and soluble lectins produced by opportunistic bacteria can also interact with FL sIg,6,7 suggesting that lectin-mediated interactions may not be limited to DC-SIGN. Thus, although the nature of lectin binding to FL cells in vivo is likely multifaceted, the findings that DC-SIGN bound specifically to FL B cells but not nonmalignant residual B cells and bound poorly to normal naive, memory, or germinal center B cells underscore this interaction as a feature that is unique to the malignant cells.
Using fluorescence microscopy and flow cytometry, Amin et al subsequently showed that DC-SIGN binding to FL cells induced organization of the BCR and CD19 into signaling platforms in a manner that is similar to antigen-mediated BCR activation in normal B cells (see figure). This caused a delayed but sustained phosphorylation of key downstream BCR signaling kinases including spleen tyrosine kinase (SYK), protein kinase B (AKT), and extracellular signal-regulated kinase (ERK). Similarly, Linley et al found that in contrast to normal B cells, exposure of FL samples to DC-SIGN triggered prolonged phosphorylation of AKT, ERK, and phospholipase C-γ-2 (PLCγ2) and increased expression of cMYC, supporting the concept that DC-SIGN is able to activate and maintain proliferation signals that could promote disease progression.
In experiments carried out by both groups, DC-SIGN–mediated BCR signaling was attenuated with inhibitors of Bruton tyrosine kinase (BTK) or SYK, revealing the therapeutic potential of the lectin interaction. Indeed, Amin et al further demonstrated that coculture of in vitro–differentiated macrophages with FL cells led to observable cell-cell interactions with coclustering of DC-SIGN on macrophages and BCR on FL cells and that blockade of these interactions with an anti-DC-SIGN antibody reduced viability of FL cells in culture.
Although Linley et al did not identify any consistent differences in substrate phosphorylation between IgM+ and IgG+ FL cells, Amin et al found that BCR activation in DC-SIGN high-binding IgM+ FL was stronger than in DC-SIGN low-binding IgM+ FL and that IgG+ FL cells were poorly activated by DC-SIGN. These discrepancies may be due to the limited number of cases analyzed as well as technical variations in the activation conditions. Nonetheless, the collective findings support a model whereby lectins within the microenvironment might foster FL cell survival and proliferation by providing a continuous activation signal through antigen-independent interactions with sIg (see figure). These studies provide a foundation for future investigations directed at understanding the role of mannosylated sIg in lymphomagenesis, determining the clinical significance of mannose glycosylation profiles in FL, and exploring targeted therapeutic approaches to disrupt glycan-lectin interactions in the microenvironment.
Conflict-of-interest disclosure: The author declares no competing financial interests.