In this issue of Blood, Ricciardi et al report a novel fatty acid oxidation (FAO) inhibitor, ST1326, that effectively inhibits proliferation, survival, and chemoresistance in leukemia cell lines and primary samples.1
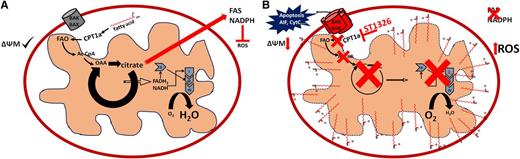
FAO supports mitochondrial function and integrity. (A) FAO supports Krebs cycle activity, electron transport, fatty acid synthesis (FAS), generation of reduced NAD phosphate (NADPH; antioxidant defense), and mitochondrial integrity in leukemia cells, resulting in an antiapoptotic phenotype. (B) Exposure to ST1326 inhibits FAO, Krebs cycle activity, FAS, and the generation of antioxidant defenses, compromising mitochondrial function. Additionally, accumulation of free palmitate perturbs mitochondrial membrane integrity (low ΔΨm), resulting in a proapoptotic phenotype that may be exacerbated by increased levels of reactive oxygen species (ROS).
FAO supports mitochondrial function and integrity. (A) FAO supports Krebs cycle activity, electron transport, fatty acid synthesis (FAS), generation of reduced NAD phosphate (NADPH; antioxidant defense), and mitochondrial integrity in leukemia cells, resulting in an antiapoptotic phenotype. (B) Exposure to ST1326 inhibits FAO, Krebs cycle activity, FAS, and the generation of antioxidant defenses, compromising mitochondrial function. Additionally, accumulation of free palmitate perturbs mitochondrial membrane integrity (low ΔΨm), resulting in a proapoptotic phenotype that may be exacerbated by increased levels of reactive oxygen species (ROS).
It has been suggested that FAO promotes leukemia stem cell survival and quiescence by supporting mitochondrial oxidative metabolism and increasing the threshold for activation of the intrinsic apoptotic pathway.2 Metabolically, FAO feeds large amounts of fatty acyl–derived acetyl-coenzyme A (CoA) into the Krebs cycle, allowing the regeneration of citrate and the continuous production of nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide to support the molecular reduction of oxygen into water (see figure). The regeneration of citrate is an obligate step for the de novo synthesis of lipid membrane components; it is essential for cellular proliferation and forms part of an apparently “futile” metabolic cycle of FAO and fatty acid synthesis that has been proposed to be another key hallmark of cancer cell metabolism.3 Although seemingly wasteful, this futile cycle is essential for the generation of antioxidant defenses3 and antagonizes the oligomerization of Bax and Bak in response to apoptotic stimuli,2 providing a fundamental barrier to cell death. Because FAO occurs in the mitochondrial matrix, which is mostly impermeable to free fatty acids or CoA esterified fatty acids, the rate-limiting step is the transfer of acyls from CoA to carnitine by the enzyme carnitine palmitoyltransferase 1 (CPT1), producing acylcarnitines. Acylcarnitines are then spontaneously translocated through the mitochondrial membrane into the matrix, where CPT2 catalyzes the re-formation of CoA esterified fatty acids to be metabolized by FAO.
ST1326 is an aminocarnitine derivative that is highly selective for the CPT1a isoform,4 a favorable characteristic when compared with etomoxir—the prototypical CPT1 inhibitor that irreversibly inhibits both a (liver) and b (muscle/heart) isoforms of the enzyme.5 Although etomoxir has been reported to efficiently induce apoptosis and sensitize leukemia cells to chemotherapy,2 its high cost and the potential on-target toxicity of inhibiting FAO in skeletal and cardiac muscle6,7 have precluded its clinical development as an antileukemic agent. In contrast, an oral formulation of ST1326 (teglicar) has been tested in phase 2 studies for the treatment of type 2 diabetes, in which it demonstrated an excellent safety profile. Importantly, Ricciardi et al demonstrate for the first time that (1) all leukemia cell lines and primary acute myeloid leukemia cells examined express the CPT1a isoform of the enzyme; (2) ST1326 potently inhibits FAO and overall mitochondrial oxygen consumption in cell lines and primary samples; and (3) ST1326 induces cell death in disease initiating but not normal bone marrow progenitors. Taken together, these findings suggest that ST1326 may be a targeted agent for the treatment of leukemia.
Mechanistically, ST1326 induces cytotoxicity via activation of the mitochondrial apoptotic pathway, as evidenced by an early drop in mitochondrial membrane potential (ΔΨm) that precedes the externalization of phosphatidyl serine and DNA fragmentation. Intriguingly, although ST1326 has been reported to be a competitive/reversible inhibitor of CPT1a,8 drug washout experiments revealed that this agent was irreversibly cytotoxic, suggesting that even transient inhibition of CPT1a-dependent FAO results in a lethal mitochondrial insult in leukemia cells. Although the precise nature of this insult is not discussed by Ricciardi et al, the authors convincingly demonstrate a marked (>6-fold) accumulation of palmitate in the cytoplasm of leukemia cells treated with ST1326, a critical finding that supports the notion that excess free fatty acids perturb mitochondrial membrane integrity and/or function, at least in part by virtue of their detergent properties that may promote the leakage of protons into the mitochondrial matrix, “short-circuiting” ΔΨm (see figure). In addition, it is tempting to speculate that, like etomoxir2 and the recently reported FAO inhibitor avocatin B,9 ST1326 may induce accumulation of ROS and/or may facilitate Bax/Bak oligomerization in the outer mitochondrial membrane. If so, BH3 mimetics such as ABT-737 may increase the therapeutic efficacy of ST1326—as they do for etomoxir in vitro and in xenograft models of AML.2
The knowledge that ST1326 imparts a rapid and irreversible insult on leukemia cells will have bearing on the design of dosing schedules in upcoming clinical trials of this agent and may provide avenues to mitigate unwanted toxicities. Moreover, the finding that ST1326 sensitizes leukemia cells to cytarabine (AraC) cytotoxicity supports the potential utility of this agent in combination with mainstay antileukemia agents or other targeted agents. In particular, it is of utmost importance to determine if ST1326 can indeed facilitate Bax/Bak oligomerization because this would provide strong rationale for combination studies with BH3 mimetics already in the clinic, such as ABT-199, a clinical derivative of ABT-737 with a more favorable toxicity profile.10 Last, although limited to findings on CD34+ myeloid leukemia progenitors, the potential ability of ST1326 to target disease-initiating cells as a single agent may warrant investigating its use as part of maintenance or stem cell transplantation regimens.
In conclusion, the results presented by Ricciardi et al in this issue of Blood uncover FAO and CPT1a as novel metabolic targets for the therapy of leukemias and demonstrate that ST1326, alone or in combination with AraC, can effectively inhibit FAO and induce cytotoxicity in leukemia cell lines and primary disease initiating progenitors. The work discussed here supports the rapid translation of teglicar (or an IV equivalent) into preclinical models of leukemia—and perhaps even into human clinical trials to determine the utility of targeting FAO for the therapy of this largely incurable group of hematological malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.