Key Points
In the absence of FXIIIa activity, red blood cells are extruded from clots during clot contraction.
Factor XIIIa promotes red blood cell retention in contracting clots by crosslinking fibrin α-chains.
Abstract
Factor XIII(a) [FXIII(a)] stabilizes clots and increases resistance to fibrinolysis and mechanical disruption. FXIIIa also mediates red blood cell (RBC) retention in contracting clots and determines venous thrombus size, suggesting FXIII(a) is a potential target for reducing thrombosis. However, the mechanism by which FXIIIa retains RBCs in clots is unknown. We determined the effect of FXIII(a) on human and murine clot weight and composition. Real-time microscopy revealed extensive RBC loss from clots formed in the absence of FXIIIa activity, and RBCs exhibited transient deformation as they exited the clots. Fibrin band-shift assays and flow cytometry did not reveal crosslinking of fibrin or FXIIIa substrates to RBCs, suggesting FXIIIa does not crosslink RBCs directly to the clot. RBCs were retained in clots from mice deficient in α2-antiplasmin, thrombin-activatable fibrinolysis inhibitor, or fibronectin, indicating RBC retention does not depend on these FXIIIa substrates. RBC retention in clots was positively correlated with fibrin network density; however, FXIIIa inhibition reduced RBC retention at all network densities. FXIIIa inhibition reduced RBC retention in clots formed with fibrinogen that lacks γ-chain crosslinking sites, but not in clots that lack α-chain crosslinking sites. Moreover, FXIIIa inhibitor concentrations that primarily block α-, but not γ-, chain crosslinking decreased RBC retention in clots. These data indicate FXIIIa-dependent retention of RBCs in clots is mediated by fibrin α-chain crosslinking. These findings expose a newly recognized, essential role for fibrin crosslinking during whole blood clot formation and consolidation and establish FXIIIa activity as a key determinant of thrombus composition and size.
Introduction
Fibrinogen is a 340-kDa plasma glycoprotein composed of 2 sets each of 3 chains (Aα, Bβ, and γ) that circulates at 2 to 4 mg/mL. During coagulation, thrombin cleaves N-terminal peptides from the Aα- and Bβ-chains, producing fibrin monomers that polymerize into protofibrils and subsequently fibers.1 Fibrinogen deficiency is associated with bleeding and/or thrombosis,2 whereas elevated fibrinogen (hyperfibrinogenemia) is associated with thrombosis.3-5
Factor XIII (FXIII) is a plasma protransglutaminase composed of 2 A (FXIII-A) and 2 B (FXIII-B) subunits that circulate as a heterotetrameric zymogen (FXIII-A2B2). FXIII activation occurs via thrombin-mediated cleavage of an N-terminal activation peptide from FXIII-A, and calcium-mediated dissociation of the inhibitory, carrier FXIII-B subunits, rendering catalytically active FXIIIa.6 FXIII deficiency is associated with frequent bruising, hematomas, miscarriage, poor wound healing, and intracranial hemorrhage.7
FXIIIa increases clot stability by introducing ε-N-(γ-glutamyl)-lysyl crosslinks between residues in the γ- and α-chains of fibrin monomers within individual fibers. Although crosslinking causes only subtle-to-no changes in fibrin network morphology,8,9 crosslinking significantly decreases the extensibility and elasticity of individual fibers and increases fibrin elastic modulus (stiffness).10-14 Interestingly, these effects are specifically associated with formation of high molecular weight (HMW) crosslinked fibrin species (γ-multimers, α-polymers, and αγ-hybrids).14-17 Studies using recombinant fibrinogen that cannot undergo γ-chain crosslinking (γQ398N/Q399N/K406R [γNNR]) reveal that fiber stiffening is primarily due to α-chain crosslinking.15-17 Crosslinking of other plasma proteins (eg, α2-antiplasmin, fibronectin) to fibrin increases the resistance of the network to biochemical degradation.6 Together, these studies demonstrate the multifunctional role of FXIIIa in clot mechanical and biochemical properties.
Recently, we showed that FXIIIa also mediates clot composition and size during whole blood clot formation.18 Using an in vitro clot contraction (also called retraction) assay, we found that human and mouse whole blood clots formed in the absence of plasma FXIII or presence of a FXIIIa inhibitor have reduced retention of red blood cells (RBCs) and are significantly smaller than normal clots. Importantly, compared with wild-type (WT) controls, FXIII-deficient mice produce smaller venous thrombi that contain significantly fewer RBCs, suggesting FXIIIa-mediated retention of RBCs in clots modulates thrombus size.18 Collectively, these results reveal a previously unrecognized function for FXIIIa activity during venous thrombosis (VT) and suggest FXIIIa is a potential therapeutic target for reducing VT. However, the mechanism by which FXIIIa mediates RBC retention in clots is not known.
Herein, we show that FXIIIa mediates RBC retention in clots specifically via its ability to crosslink fibrin α-chains. These results define new (patho)physiologic roles for FXIIIa activity and fibrin α-chain crosslinking in hemostasis and thrombosis.
Methods
Proteins and materials
Sources are detailed in the supplemental Methods available on the Blood Web site. Phlebotomy was approved by the University of North Carolina and Georgia Institute of Technology Institutional Review Boards and performed on consenting donors in accordance with the Declaration of Helsinki. Murine studies were approved by the University of Amsterdam Academic Medical Center Animal Care and Use Committee and St. Michael’s Hospital Animal Care Committee.
Human blood clot contraction
Clot contraction assays were performed as previously described.18 Briefly, blood was drawn from healthy donors via venipuncture and added to siliconized wells of a 96-well plate containing tissue factor (TF, 1 pM, final), CaCl2 (10 mM, final), and the FXIIIa active site inhibitor, T101 (10 μM, final) or HEPES-buffered saline (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 150 mM NaCl, pH 7.4). Clot formation and contraction were allowed to proceed for 90 minutes at 37°C. Serum RBC content was measured by absorbance (575 nm) with a SpectraMax Plus 340 plate reader (Molecular Devices) and compared with the initial absorbance. Clots were weighed and/or prepared for microscopy.
For experiments using recombinant fibrinogen, blood was drawn from a fibrinogen-deficient individual (<40 mg/dL fibrinogen, infinite thrombin clotting time; supplemental Figure 1) and processed to platelet-rich plasma (PRP) by centrifugation (150g, 20 minutes). PRP was then processed to platelet-poor plasma (PPP) by centrifugation (1500g, 10 minutes). PPP was flash-frozen in liquid nitrogen and stored at −80°C. Platelets and RBCs were isolated from healthy, consenting, blood type O-negative donors as previously described18 and counted on a pocH-100i Hematology Analyzer (Sysmex). Platelets (200 000/μL, final) and RBCs (2 million/μL, final) were then added to thawed fibrinogen-deficient plasma and supplemented with recombinant fibrinogen (0.25 mg/mL, final in plasma fraction, unless otherwise noted) before clot contraction was initiated with TF (1 pM, final) and recalcification (10 mM, final).
Microscopy
For real-time confocal microscopy of contracting clots, RBCs were isolated from whole blood and fluorescently labeled with octadecyl rhodamine B chloride. Alexa Fluor 488-labeled fibrinogen (75 μg/mL, final) and labeled RBCs (10%, final) were added to whole blood, and clotting was triggered with TF (1 pM, final) and recalcification (10 mM, final) in siliconized glass-bottom petri dishes at 37°C and 60% humidity. Reactions were performed in the presence of the fibrinolysis inhibitor ε-aminocaproic acid (ε-ACA, 5 mM, final), in the absence and presence of T101.
For real-time difference interference contrast microscopy, clot contraction in whole blood was triggered in a polydimethylsiloxane microfluidics device with thrombin (1 U/mL [10 nM], final), CaCl2 (10 mM, final), and T101 (500 μM, final).
Fibrin band-shift assay
Citrated whole blood or PRP was clotted with thrombin (20 nM, final) and recalcification (5 mM CaCl2, final) in the absence or presence of T101 (200 μM, final) for 2 hours at 37°C. Clots were then separated from serum by centrifugation (250g, 15 minutes). Cells were lysed, and clots washed by incubation in 1× Cell Lysis Buffer (Cell Signaling) with 1 mM phenylmethylsulfonyl fluoride (MP Biomedicals), followed by homogenization and washing with distilled water. Clots were subsequently dissolved in 50 mM dithiothreitol/12.5 mM EDTA in 8 M urea (dithiothreitol/EDTA/urea) at 60°C for 1 hour. Samples were boiled in sodium dodecyl sulfate (SDS)-containing sample buffer and separated using SDS-polyacrylamide gel electrophoresis (PAGE) on 10% Tris-glycine gels.
FXIIIa substrate crosslinking to RBCs
RBCs were isolated from citrated whole blood by centrifugation (150g, 20 minutes), washed in citrate-glucose-saline, and packed as previously described.18 Packed RBCs (10 μL) were incubated with a Cy5-labeled peptide derived from the amino terminus of α2-antiplasmin (A15, Ac-GNQEQVSPLTLLKWC[Cy5]-NH2, 3 μM, final)21 or biotinylated cadaverine (BC, 5 mM, final) in the presence of FXIII-A2B2 (20 μg/mL, final), thrombin (5 nM, final), and CaCl2 (10 mM, final), rotating for 1 hour (40 µL, final volume). Control samples contained 0.5 mg/mL Peak 1 fibrinogen and no RBCs. For SDS-PAGE, reactions were quenched and dissolved in dithiothreitol/EDTA/urea, boiled, and separated on 10% Tris-glycine gels. A15-crosslinked species were directly visualized on a GE Typhoon FLA-9000 Imager (GE Healthcare). Gels of BC samples were transferred to polyvinylidene fluoride (PVDF) membranes and probed with 0.2 μg/mL Alexa Fluor 488-labeled streptavidin before visualization on the Typhoon Imager. For flow cytometry, samples were diluted 1:200 in flow buffer (21 mM Tris [pH 7.4], 140 mM NaCl, 11.1 mM dextrose, 4.7 mM KCl, 1.2 mM MgSO4, and 0.1% PEG 8000) and labeled with phycoerythrin-labeled anti-CD235a. Samples containing BC were also labeled with Alexa Fluor 488-labeled streptavidin. Samples were diluted in flow buffer and analyzed using a Stratedigm S1000Ex flow cytometer.
Murine blood clot contraction
Blood was drawn into citrate from the inferior vena cava of anesthetized WT, α2-antiplasmin–deficient,22 and thrombin-activatable fibrinolysis inhibitor (TAFI)-deficient23 mice, or Fnfl/flMx Cre− (pFn+) and Fnfl/flMx Cre+(pFn−) mice.24 Plasma fibronectin depletion was induced by 3 intraperitoneal injections of 250 μg polyinonic-polycytidylic acid (Sigma) into Cre+ and Cre− littermates at 2-day intervals. Depletion of pFn in plasma and platelets was confirmed by western blotting. For WT, α2-antiplasmin–deficient, and TAFI-deficient mice, blood was pooled from 6 to 9 mice before clotting. For pFn+ and pFn− mice, blood from 6 to 9 separate mice was studied. Blood was added whole or diluted 1:3 in HEPES-buffered saline to siliconized tubes containing TF (1 pM, final) and CaCl2 (10 mM, final), as previously described.18 FXIIIa was inhibited with T101 (5 μM, final). Serum RBC content was measured by absorbance (575 nm).
Isolation of differentially aged RBC populations
RBCs were age separated using density gradient centrifugation25 (detailed in supplemental Methods).
Recombinant fibrinogen generation
Recombinant WT (γA/γA)26 fibrinogen or variants, fibrinogen with mutations that remove γ-chain crosslinking sites (γQ398N/Q399N/K406R, γNNR15 ) or truncate the α-chain (Aα25127 ), were expressed and purified as described in the supplemental Methods. Compared with γA/γA fibrinogen, recombinant fibrinogen variants clotted with similar onset times and rates, as expected.17,27
Western blotting
Clots were dissolved in dithiothreitol/EDTA/urea (60°C, 1 hour), boiled in SDS-containing sample buffer, and separated using SDS-PAGE on 10% Tris-glycine gels before transfer to PVDF membranes. Densitometry was performed using ImageJ 1.48v. Band intensity of HMW species and γ-γ dimers was normalized to the β-chain before normalization to untreated controls.
Statistical methods
Serum RBC content and clot weights were compared using paired or 1-tailed Student t tests, as appropriate. Significance of correlations was determined by Pearson’s correlation coefficient. Percent changes in clot weight, network density, fiber thickness, probe crosslinking to RBCs, and IC50 values were compared using an unpaired, 2-tailed Student t test. P < .05 was considered statistically significant.
Results
In the absence of FXIIIa activity, RBCs are extruded from the clot during clot contraction
We previously showed that FXIIIa promotes RBC retention in contracting clots.18 To visualize this process, we generated whole blood clots in the presence of Alexa Fluor 488-labeled fibrinogen, octadecyl rhodamine B chloride-labeled RBCs, and ε-ACA (to inhibit fibrinolysis), in the absence and presence of the FXIIIa inhibitor, T101. We then visualized clot contraction using real-time confocal and differential interference contrast microscopy (Figure 1). In clots with normal FXIIIa activity, most RBCs were retained within the clot (Figure 1A; supplemental Video 1). In contrast, inhibiting FXIIIa with T101 increased RBC mobility within the contracting clot and increased RBC extrusion from the clot (Figure 1B; supplemental Video 2). In some clots, we observed “plumes” of RBCs being ejected from the clot (supplemental Video 3). In both cases, RBCs exiting the clots exhibited substantial, but transient, deformation, consistent with their highly deformable nature (Figure 1C). Notably, RBCs resumed their characteristic discoid shape once free of the clot (Figure 1C). Together with previous findings,18 these images demonstrate a critical role for FXIIIa activity in RBC retention in clots.
In the absence of FXIIIa activity, RBCs are extruded from the clot during clot contraction. (A-B) Clot formation and contraction were triggered by recalcification (10 mM, final) and addition of TF (1 pM, final) to whole blood spiked with octadecyl rhodamine B-labeled RBCs, Alexa Fluor 488-labeled fibrinogen, and ε-ACA (to inhibit fibrinolysis). Clot contraction was visualized on a Zeiss 710 NLO confocal laser scanning microscope with an incubation chamber and temperature stage (Carl Zeiss, Thornwood, NY), and monitored at 40× magnification. Representative frames of contracting clots formed in the (A) absence and (B) presence of T101. Times (in seconds) are indicated in each panel. Scale bar, 50 μm. (C) Clot formation and contraction was triggered by addition of thrombin (1 U/mL [10 nM], final) and recalcification (10 mM, final) to whole blood. Clot contraction was visualized at 10× with digital zoom on a Nikon Eclipse TE2000-U inverted microscope (Nikon Instruments, Melville, NY). Frames depict RBC extrusion from a contracting clot. Black arrowheads highlight a deforming RBC as it exits the clot. Numbers indicate successive frames. Scale bar, 10 μm.
In the absence of FXIIIa activity, RBCs are extruded from the clot during clot contraction. (A-B) Clot formation and contraction were triggered by recalcification (10 mM, final) and addition of TF (1 pM, final) to whole blood spiked with octadecyl rhodamine B-labeled RBCs, Alexa Fluor 488-labeled fibrinogen, and ε-ACA (to inhibit fibrinolysis). Clot contraction was visualized on a Zeiss 710 NLO confocal laser scanning microscope with an incubation chamber and temperature stage (Carl Zeiss, Thornwood, NY), and monitored at 40× magnification. Representative frames of contracting clots formed in the (A) absence and (B) presence of T101. Times (in seconds) are indicated in each panel. Scale bar, 50 μm. (C) Clot formation and contraction was triggered by addition of thrombin (1 U/mL [10 nM], final) and recalcification (10 mM, final) to whole blood. Clot contraction was visualized at 10× with digital zoom on a Nikon Eclipse TE2000-U inverted microscope (Nikon Instruments, Melville, NY). Frames depict RBC extrusion from a contracting clot. Black arrowheads highlight a deforming RBC as it exits the clot. Numbers indicate successive frames. Scale bar, 10 μm.
FXIIIa does not crosslink RBCs to fibrin
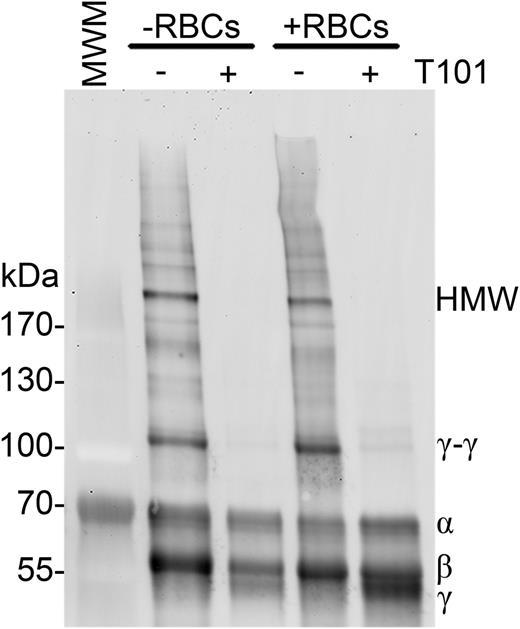
We first tested the hypothesis that FXIIIa directly crosslinks RBCs to fibrin. Although RBCs noncovalently bind fibrin(ogen),28-30 a covalent interaction between RBCs and fibrin has not been identified. To determine whether FXIIIa crosslinks a RBC surface protein to fibrin, we analyzed fibrin in clots generated in the absence and presence of RBCs and FXIIIa activity. We anticipated that if FXIIIa crosslinks RBCs to fibrin, crosslinked fibrin formed in the presence of RBCs would migrate more slowly than fibrin formed in the absence of RBCs. Figure 2 shows that clots formed in the presence of RBCs did not exhibit fibrin band shifts or new bands relative to clots formed in the absence of RBCs. These data are consistent with prior studies that identified transglutaminase-2 substrates on the cytoplasmic, but not outer, surface of the RBC membrane31,32 and suggest FXIIIa does not crosslink RBCs to fibrin.
FXIIIa does not crosslink fibrin to RBCs. Clotting was initiated in recalcified (5 mM, final) PRP (−RBCs) or whole blood (+RBCs) with thrombin (20 nM, final), in the absence or presence of T101 (200 μM, final). Clots were then dissolved and fibrin crosslinking patterns were analyzed by western blotting using a polyclonal anti-human fibrinogen antibody. Representative blot of n = 4 experiments.
FXIIIa does not crosslink fibrin to RBCs. Clotting was initiated in recalcified (5 mM, final) PRP (−RBCs) or whole blood (+RBCs) with thrombin (20 nM, final), in the absence or presence of T101 (200 μM, final). Clots were then dissolved and fibrin crosslinking patterns were analyzed by western blotting using a polyclonal anti-human fibrinogen antibody. Representative blot of n = 4 experiments.
FXIIIa does not crosslink glutamine- or lysine-reactive substrates to RBCs
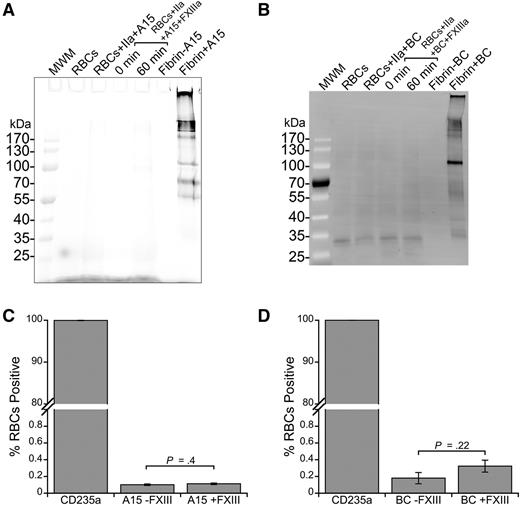
To identify any fibrin-independent FXIIIa substrates on RBCs, we used 2 probes for FXIIIa activity: A15 peptide and BC. A15 peptide contains a glutamine residue recognized by FXIIIa21 and BC mimics a reactive lysine substrate of FXIIIa.33 Whereas FXIIIa efficiently crosslinked A15 (Figure 3A) and BC (Figure 3B) to fibrin, analysis of cell lysates of washed RBCs incubated with FXIIIa and A15 or BC did not reveal any labeled proteins (Figure 3A-B; lower limit of detection, ∼300 molecules per cell, data not shown).
FXIIIa does not crosslink FXIIIa substrates to RBCs. (A) Cy5-labeled A15 peptide (glutamine donor, 3 μM, final) or (B) biotinylated cadaverine (BC, lysine acceptor, 5 mM, final) was incubated with FXIII (20 μg/mL, final) and washed RBCs in the presence of thrombin (IIa, 5 nM, final) and CaCl2 (10 mM, final) to probe for reactive lysine and glutamine residues, respectively, on the RBC surface. RBCs were then lysed, proteins were separated by SDS-PAGE, and labeling was visualized using Cy5 fluorescence (A15) or by transfer to a PVDF membrane and probing with Alexa Fluor 488-labeled streptavidin. Control reactions contained fibrinogen (0.5 mg/mL, final), FXIII, thrombin, CaCl2, and A15 or BC. (C-D) For flow cytometry, intact cells were incubated with a phycoerythrin-labeled anti-human CD235a antibody and analyzed for (C) A15 and (D) BC labeling. Bars are means ± standard error (SE).
FXIIIa does not crosslink FXIIIa substrates to RBCs. (A) Cy5-labeled A15 peptide (glutamine donor, 3 μM, final) or (B) biotinylated cadaverine (BC, lysine acceptor, 5 mM, final) was incubated with FXIII (20 μg/mL, final) and washed RBCs in the presence of thrombin (IIa, 5 nM, final) and CaCl2 (10 mM, final) to probe for reactive lysine and glutamine residues, respectively, on the RBC surface. RBCs were then lysed, proteins were separated by SDS-PAGE, and labeling was visualized using Cy5 fluorescence (A15) or by transfer to a PVDF membrane and probing with Alexa Fluor 488-labeled streptavidin. Control reactions contained fibrinogen (0.5 mg/mL, final), FXIII, thrombin, CaCl2, and A15 or BC. (C-D) For flow cytometry, intact cells were incubated with a phycoerythrin-labeled anti-human CD235a antibody and analyzed for (C) A15 and (D) BC labeling. Bars are means ± standard error (SE).
Because RBC interactions with proteins can be mediated by cell membrane lipids, including sulfatide,34 we also incubated washed RBCs with FXIIIa and A15 peptide or BC and analyzed intact cells using flow cytometry. Similar to findings with SDS-PAGE, we did not detect A15 peptide or BC on intact CD235a-positive cells (RBCs) in the absence or presence of FXIIIa, suggesting RBCs do not have ligands for these established FXIIIa substrates (Figure 3C-D). Collectively, these findings indicate FXIIIa does not promote RBC retention by crosslinking RBCs into the clot.
α2-Antiplasmin, TAFI, and fibronectin are not required for FXIIIa-mediated RBC retention
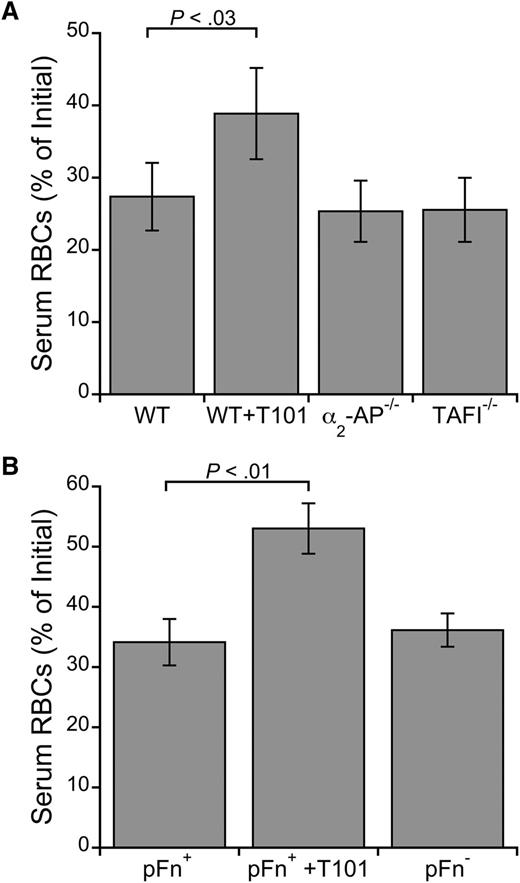
Three established nonfibrin substrates of FXIIIa are α2-antiplasmin, TAFI, and fibronectin. We therefore tested whether these proteins are required for RBC retention by analyzing clot contraction of whole blood from mice deficient in these proteins. Although FXIIIa inhibition increased RBC extrusion from WT mouse clots (P < .03; Figure 4A-B), deficiency in α2-antiplasmin, TAFI, or fibronectin did not increase RBC loss (Figure 4A-B). Moreover, RBC loss was similar in WT blood clotted in the absence and presence of the fibrinolysis inhibitor ε-ACA (serum RBC content was 31.6% and 32.5% of initial, respectively). Together, these results show that these canonical FXIIIa substrates are not required for RBC retention in clots and that the effect of FXIIIa is not due to its antifibrinolytic function.
FXIIIa does not require α2-antiplasmin, TAFI, or fibronectin to promote RBC retention in clots. Serum RBC content from ex vivo clot contraction assays using whole blood from (A) WT, α2-antiplasmin–deficient (α2-AP−/−), and TAFI-deficient (TAFI−/−) mice (n = 3) or (B) fibronectin-sufficient (pFn+) and -deficient (pFn−) mice (n = 6-9). Clotting was initiated with TF (1 pM, final) and recalcification (10 mM, final). WT and pFn+ blood was treated with T101 (5 μM, final) as positive controls. Bars are means ± SE.
FXIIIa does not require α2-antiplasmin, TAFI, or fibronectin to promote RBC retention in clots. Serum RBC content from ex vivo clot contraction assays using whole blood from (A) WT, α2-antiplasmin–deficient (α2-AP−/−), and TAFI-deficient (TAFI−/−) mice (n = 3) or (B) fibronectin-sufficient (pFn+) and -deficient (pFn−) mice (n = 6-9). Clotting was initiated with TF (1 pM, final) and recalcification (10 mM, final). WT and pFn+ blood was treated with T101 (5 μM, final) as positive controls. Bars are means ± SE.
Fibrin network density mediates RBC retention in clots, but effects of FXIIIa are independent of fibrin density
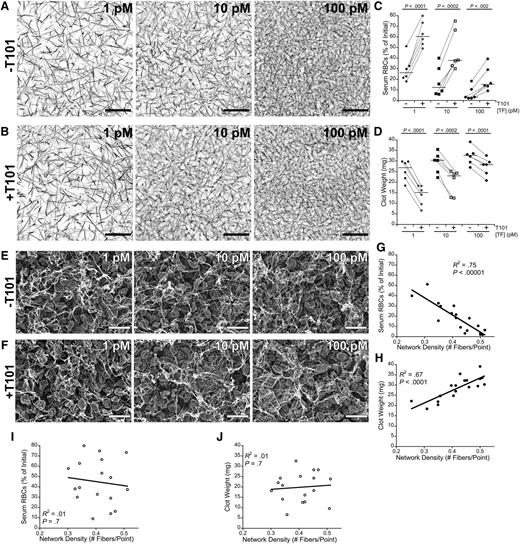
RBC retention in clots has traditionally been attributed to steric effects of the fibrin network. Therefore, we examined the effect of fibrin network density on RBC retention by triggering clot formation using a range of TF concentrations (1, 10, and 100 pM, final) to produce clots with different initial network densities20 in the absence and presence of FXIIIa activity (±T101) (Figure 5A-B). As expected, increasing the TF concentration increased fibrin network density in both the absence and presence of FXIIIa activity, and at each TF concentration, clots that formed in the absence and presence of FXIIIa activity had similar network densities. We then correlated the TF concentration with the serum RBC content and weight of fully contracted whole blood clots. As predicted, increasing the concentration of TF decreased serum RBC content and increased clot weight (Figure 5C-D). These data indicate fibrin network density influences RBC retention but that FXIIIa does not promote RBC retention in clots by increasing fibrin network density. Notably, at all TF concentrations tested, FXIIIa inhibition increased RBC extrusion from clots two- to fourfold (P < .002; Figure 5C) and decreased clot weight 16% to 48% (P < .0002; Figure 5D), indicating an independent effect of FXIIIa.
Fibrin network density promotes RBC retention in clots, but FXIIIa mediates RBC retention independent of fibrin network density. (A-B) Recalcified (10 mM, final) plasma spiked with Alexa Fluor 647-labeled fibrinogen was clotted with the indicated TF concentrations in the (A) absence or (B) presence of T101. Images are representative confocal micrographs (z-projections of 30 individual slices) of clots visualized on a Zeiss LSM710 laser scanning confocal microscope with a 63× oil-immersion lens (Carl Zeiss). Scale bar, 30 μm. (C) Serum RBC content and (D) clot weight following clot contraction. Each dot represents an individual clot. Lines connect clots formed from the same blood donor. Horizontal dark lines indicate medians. (E-F) Representative scanning electron micrographs of clots formed in recalcified (10 mM, final) whole blood with the indicated TF concentrations in the (E) absence and (F) presence of T101 (10 μM, final). Clots were visualized at 5010× on a Zeiss Supra 25 Field Emission Scanning Electron Microscope (Carl Zeiss). Scale bar, 10 μm. Micrographs were used to measure fibrin network density (supplemental Methods), which was compared with the (G,I) serum RBC content or (H,J) clot weight following clot contraction in the (G-H) absence and (I-J) presence of T101.
Fibrin network density promotes RBC retention in clots, but FXIIIa mediates RBC retention independent of fibrin network density. (A-B) Recalcified (10 mM, final) plasma spiked with Alexa Fluor 647-labeled fibrinogen was clotted with the indicated TF concentrations in the (A) absence or (B) presence of T101. Images are representative confocal micrographs (z-projections of 30 individual slices) of clots visualized on a Zeiss LSM710 laser scanning confocal microscope with a 63× oil-immersion lens (Carl Zeiss). Scale bar, 30 μm. (C) Serum RBC content and (D) clot weight following clot contraction. Each dot represents an individual clot. Lines connect clots formed from the same blood donor. Horizontal dark lines indicate medians. (E-F) Representative scanning electron micrographs of clots formed in recalcified (10 mM, final) whole blood with the indicated TF concentrations in the (E) absence and (F) presence of T101 (10 μM, final). Clots were visualized at 5010× on a Zeiss Supra 25 Field Emission Scanning Electron Microscope (Carl Zeiss). Scale bar, 10 μm. Micrographs were used to measure fibrin network density (supplemental Methods), which was compared with the (G,I) serum RBC content or (H,J) clot weight following clot contraction in the (G-H) absence and (I-J) presence of T101.
We also measured final fibrin network density in fully contracted whole blood clots. As seen in studies of plasma clots,8,9 the absence or presence of FXIIIa activity (±T101) did not significantly alter fibrin network density (P > .07) or fiber thickness (P > .10) at any TF concentration tested (Figure 5E-F). In the presence of FXIIIa activity, final fibrin network density was strongly, negatively correlated with serum RBC content (Figure 5G) and strongly, positively correlated with final clot weight (Figure 5H). Interestingly, however, these correlations were lost in the presence of T101 (Figure 5I-J), suggesting substantial structural heterogeneity arises in the fibrin network during the extensive RBC loss and clot contraction that occur in the absence of FXIIIa.
FXIIIa inhibition reduces RBC retention in clots lacking γ-, but not α-chain, crosslinking
Finally, we tested the hypothesis that FXIIIa promotes RBC retention in clots via its ability to crosslink fibrin. FXIIIa crosslinks residues between γ- and α-chains within fibrin fibers, and studies have revealed distinct effects of each type of crosslinking on the fibrin network.14-17,35 Therefore, we specifically interrogated the individual contributions of γ- and α-chain crosslinks to RBC retention.
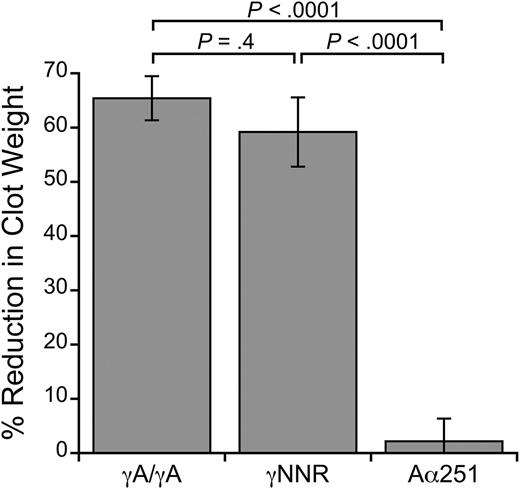
We first performed clot contraction assays using recombinant fibrinogens mutated to eliminate either γ- or α-chain crosslinking (supplemental Figure 2). To test the role of γ-chain crosslinking, we used a fibrinogen variant (γQ398N/Q399N/K406R, γNNR15 ) that lacks the 3 γ-chain crosslinking residues. To test the role of α-chain crosslinking, we used fibrinogen with an Aα-chain truncation at residue 251 (Aα25127 ), which eliminates most of the α-chain crosslinking residues. We reconstituted fibrinogen-deficient plasma with RBCs, platelets, and either normal (WT) recombinant fibrinogen (γA/γA), γNNR, or Aα251 in the absence and presence of T101. Figure 6 shows the percent reduction in clot weight caused by the presence of T101 for each variant. FXIIIa inhibition significantly reduced the size of clots formed with γA/γA fibrinogen (3.3 ± 0.5 vs 1.2 ± 0.3 mg, respectively, P < .001) and γNNR (3.7 ± 0.5 vs 1.6 ± 0.4 mg, respectively, P < .01). These data indicate γ-chain crosslinking is not required for RBC retention in contracted clots. In contrast, inhibiting FXIIIa in clots formed with Aα251 did not reduce clot size (7.9 ± 0.6 vs 7.7 ± 0.6 mg, respectively, P = .3). Because clots formed from Aα251 were larger than those formed from γA/γA and γNNR fibrinogens, likely due to the abnormal fibrin network structure previously reported for this variant,35 we also measured RBC retention in clots formed from lower concentrations of Aα251 fibrinogen (0.125 mg/mL, final). Whereas reducing the fibrin concentration reduced clot weight, these clots still failed to show an effect of FXIIIa inhibition on clot weight (data not shown).
FXIIIa inhibition does not reduce RBC retention in clots formed with Aα251 fibrinogen. Percent reduction in clot weight of contracted clots formed from TF-treated, recalcified fibrinogen-deficient plasma reconstituted with RBCs and platelets (2 million/μL and 200 000/μL, respectively), and γA/γA, γNNR, or Aα251 fibrinogen (0.25 mg/mL, final), in the absence or presence of 10 μM (final) T101 (n = 3-7 per fibrinogen). Bars are means ± SE.
FXIIIa inhibition does not reduce RBC retention in clots formed with Aα251 fibrinogen. Percent reduction in clot weight of contracted clots formed from TF-treated, recalcified fibrinogen-deficient plasma reconstituted with RBCs and platelets (2 million/μL and 200 000/μL, respectively), and γA/γA, γNNR, or Aα251 fibrinogen (0.25 mg/mL, final), in the absence or presence of 10 μM (final) T101 (n = 3-7 per fibrinogen). Bars are means ± SE.
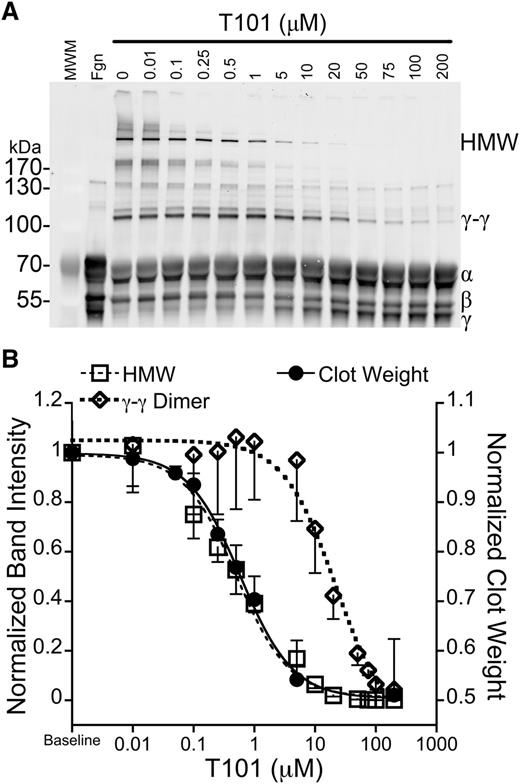
To further test the contributions of γ- and α-chain crosslinking in RBC retention, we exploited previous observations that low concentrations of FXIIIa inhibitors selectively inhibit formation of HMW α-chain–rich crosslinked species without reducing γ-γ dimer formation.14,36 We generated plasma clots in the presence of a range of T101 concentrations (0-200 μM), dissolved the clots, and probed for fibrin crosslinking via western blotting (Figure 7A). Consistent with the previous studies,14,36 T101 inhibited HMW species formation at ∼40-fold lower concentrations than it inhibited γ-γ dimer formation (IC50 = 0.52 ± 0.12 vs 21.1 ± 5.8 μM, respectively, P < .03; Figure 7B). Importantly, when we superimposed the effects of T101 on fibrin crosslinking, clot weight, and RBC retention, we found that T101 reduced clot weight (IC50 = 0.60 ± 0.09 µM; Figure 7B) and RBC retention (data not shown) at the same concentrations at which it inhibited HMW species formation (Figure 7B). Together, these data show FXIIIa promotes RBC retention in clots by crosslinking fibrin α-chains.
RBC retention is reduced at concentrations of T101 that inhibit α-chain crosslinking. (A) Recalcified (10 mM, final) plasma was clotted with TF (1 pM) in the presence of increasing concentrations of T101. Clots were dissolved and analyzed by western blotting with polyclonal anti-human fibrinogen antibody. The second lane (Fgn) is unclotted plasma. (B) Normalized intensity of γ-γ dimer (open diamonds, short dashed line, n = 3) or HMW bands (open squares, long dashed line, n = 3) superimposed on normalized clot weight following clot contraction in the presence of T101 (closed circles, solid line, n = 5-7). Data are means ± SE.
RBC retention is reduced at concentrations of T101 that inhibit α-chain crosslinking. (A) Recalcified (10 mM, final) plasma was clotted with TF (1 pM) in the presence of increasing concentrations of T101. Clots were dissolved and analyzed by western blotting with polyclonal anti-human fibrinogen antibody. The second lane (Fgn) is unclotted plasma. (B) Normalized intensity of γ-γ dimer (open diamonds, short dashed line, n = 3) or HMW bands (open squares, long dashed line, n = 3) superimposed on normalized clot weight following clot contraction in the presence of T101 (closed circles, solid line, n = 5-7). Data are means ± SE.
Discussion
The importance of FXIII activity in stabilizing clots is well established. However, the recent recognition of FXIII’s essential role in determining thrombus composition and size18 raises important questions about its contributions to clot formation in vivo. Our study to determine the mechanism mediating RBC retention in clots reveals previously unrecognized functions for FXIIIa activity and fibrin crosslinking. We showed RBC retention is mediated by fibrin network density and FXIIIa activity. However, effects of FXIIIa are independent of fibrin density, and FXIIIa does not crosslink RBCs to the clot. Importantly, we found that FXIIIa crosslinking of fibrin α-chains promotes RBC retention in clots. Although previous studies have documented unique, independent contributions of α- and γ-chain crosslinking to fibrin biophysical characteristics,14-17,35 our results are the first to associate these characteristics with a specific function during thrombus formation in vivo and demonstrate an essential role of crosslinked fibrin in determining thrombus cellular content. The findings presented here define the mechanism by which FXIIIa mediates thrombus composition and expose a newly recognized, essential (patho)physiologic function for fibrin crosslinking.
It has traditionally been thought that the fibrin network functions like a net that traps and retains RBCs flowing by the developing clot. Our findings support, but substantially refine, this concept. We observed that in a normally crosslinked clot, RBC retention is strongly associated with fibrin network density. Fibrin network density is mediated by several factors, including the concentrations of fibrinogen and thrombin present during fibrin formation.37 We and others have shown that plasma hypercoagulability results in the formation of clots with high fibrin network density.38-43 It is tempting to speculate that these dense networks trap more RBCs, resulting in large, occlusive venous thrombi. Indeed, we previously observed that hyperprothrombinemia leads to the formation of clots with increased network density44 and that mice with elevated prothrombin produce significantly larger venous thrombi38 that have higher RBC content (M. M. Aleman and A.S.W., unpublished observations, October 31, 2012). Together, these findings suggest an abnormally high density of crosslinked fibrin promotes venous occlusion by increasing RBC retention in the thrombus, and therefore, thrombus size.
Because we and others have observed little-to-no effect of fibrin crosslinking on network density,8,9 increased RBC extrusion from uncrosslinked clots is unlikely to be due to abnormal network density. Rather, these data support the premise that FXIIIa’s effects on clot biophysical properties mediate RBC retention in the clots. Previous studies have demonstrated distinct contributions of γ- and α-chain crosslinking to fibrin viscoelastic properties. Using a synthetic inhibitor and patient-derived anti-FXIII antibody, Ryan et al14 showed that the FXIIIa-mediated increase in clot stiffness correlates with the generation of α-chain-rich HMW species and not with γ-γ dimers. Furthermore, only partial α-chain crosslinking was required to reach near-maximal clot stiffness,14 consistent with our observation that even partial crosslinking of α-monomers increased RBC retention. More recently, Collet et al used recombinant Aα251 fibrinogen to show that clots with only γ-chain crosslinking are nearly 4 times less stiff than γ- and α-chain crosslinked clots.35 Similarly, studies using γNNR fibrin (only α-chain crosslinking) showed the majority of the stiffness of fully crosslinked clots is provided by α-chain crosslinking.15-17 Consequently, our observation that α-chain crosslinking mediates FXIIIa-mediated RBC retention in clots identifies a potential (patho)physiologic consequence of α-chain crosslinking and fibrin fiber stiffening during clot formation in vivo.
The observation that γ- and α-chain crosslinking is inhibited by different concentrations of inhibitor14,36 may suggest a conformational change in FXIIIa facilitates γ- vs α-chain crosslinking and that the α-chain–specific conformation is more susceptible to inhibition than the γ-specific conformation. Alternately, the spatial relationship between FXIIIa and the γ- and α-chains during FXIII activation may contribute to differential timing and inhibition of γ- and α-chain crosslinking. We recently localized FXIII zymogen binding to the homologous murine γ-chain residues 390 to 396,18 which would conveniently position activated FXIIIa near the γ-chain crosslinking sites (γ398/399/406). Thus, the rate of γ-chain crosslinking may exceed α-chain crosslinking due to the proximity of FXIII(a) to the γ-chain after activation. Whether these differences can be exploited to evaluate γ- vs α-chain crosslinking in vivo remains to be determined.
Although previous studies demonstrated specific interactions between fibrin and RBCs,28-30 and implicate CD4745 and a β3-like molecule46 on the RBC surface, we were unable to detect any FXIIIa substrates on the RBC surface. We were also unable to detect differences in retention of young vs old RBCs in clots (data not shown), indicating age-dependent changes in RBC protein expression or morphology47 do not influence their presence in clots. These data suggest FXIIIa does not covalently ligate RBCs to the clot. This finding is somewhat surprising, because a recent report suggested FXIIIa is highly promiscuous, with >147 potential substrates in plasma.48 However, our findings are consistent with a previous study that also did not identify any transglutaminase substrates on the RBC surface.31 Together, these results indicate FXIIIa retains considerable substrate specificity during coagulation in whole blood.
The consequences of RBC retention in clots is an area of active investigation. Clot contraction induces the appearance of compacted RBCs (so-called “polyhedrocytes”).49 This observation demonstrates substantial platelet-mediated forces are transmitted through the clot during contraction50,51 and suggests the fibrin network must be sufficiently dense and stiff to retain RBCs during this process. The contribution of crosslinking to fibrin elasticity, extensibility, and stiffness occurs at both whole-clot and single-fiber scales.10-14 In addition to clot contraction, shear stress from blood flow in vivo also exerts considerable force onto the fibrin network. Thus, in the absence of FXIIIa-mediated crosslinking, fibrin may deform or even break, permitting RBCs to escape. Further studies examining the contributions of crosslinked fibrin, as well as platelet contractile force and RBC deformability, to clot formation are needed to fully define the how α-chain crosslinking mediates RBC retention in clots.
This study has potential limitations. First, although we did not identify FXIIIa substrates on the RBC surface, our lower limit of detection was ∼300 molecules per cell. However, it would be surprising if a protein present at lower copy number could effectively promote RBC retention within the clot. Second, fibrin crosslinking could generate a neoepitope that promotes RBC binding and retention; however, we did not observe substantial differences in RBC binding to fibrin in the absence and presence of FXIIIa. Third, Aα251 exhibits slightly delayed crosslinking,35 which may have reduced the effect of FXIIIa. However, results from experiments with recombinant fibrinogens were consistent with findings using full-length fibrinogen in plasma and support the conclusion that α-chain crosslinking mediates RBC retention. Finally, our experiments were performed under static conditions. Although stasis recapitulates aspects of VT,52 other mechanisms may mediate RBC content in clots formed under higher (arterial) shear.
In summary, our results show FXIIIa promotes RBC retention in clots by crosslinking fibrin. Formation of α-chain–rich, HMW crosslinked fibrin species correlated strongly with RBC retention in clots, suggesting biophysical strengthening of the fibrin network secondary to α-chain crosslinking determines clot composition. Overall, these findings refine our understanding of the FXIII-fibrinogen axis and establish α-chain crosslinking as a critical determinant of thrombus formation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Caroline Schuerger, Anthony Boutelle, LiFang Ping, Arnoud Marquart, and Veronique Knaup for excellent technical assistance; Thomas Neish and the University of North Carolina (UNC) Platelet Donation Center for assistance with RBC collection; the UNC Microscopy Services Laboratory for assistance with microscopy; and Maria M. Aleman for reading the manuscript.
This study was supported by funding from National Institutes of Health National Heart, Lung, and Blood Institute grants R01HL094740 and R56HL094740 (to A.S.W.), 1UL1TR001111 (to UNC/A.S.W.), and K12HL087097-06 (to UNC/M.J.M.), National Science Foundation Graduate Research fellowship DGE-1144081 (to J.R.B.), British Heart Foundation grant RG/13/3/30104 (to R.A.S.A.), and the PhD Graduate Fellowship from Canadian Blood Services and Meredith & Malcolm Silver Scholarship in Cardiovascular Studies (to Y.W.).
Authorship
Contribution: J.R.B. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; C.D. contributed vital reagents; Y.W., C.E.H., B.A., M.J.M., and M.A.C. performed experiments; J.M.J. designed experiments; S.T.L. and R.A.S.A. provided vital reagents; W.A.L., J.C.M.M., and H.N. designed experiments; A.S.W. designed the research, analyzed and interpreted the data, and wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 819 Brinkhous-Bullitt Building, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.

![Figure 1. In the absence of FXIIIa activity, RBCs are extruded from the clot during clot contraction. (A-B) Clot formation and contraction were triggered by recalcification (10 mM, final) and addition of TF (1 pM, final) to whole blood spiked with octadecyl rhodamine B-labeled RBCs, Alexa Fluor 488-labeled fibrinogen, and ε-ACA (to inhibit fibrinolysis). Clot contraction was visualized on a Zeiss 710 NLO confocal laser scanning microscope with an incubation chamber and temperature stage (Carl Zeiss, Thornwood, NY), and monitored at 40× magnification. Representative frames of contracting clots formed in the (A) absence and (B) presence of T101. Times (in seconds) are indicated in each panel. Scale bar, 50 μm. (C) Clot formation and contraction was triggered by addition of thrombin (1 U/mL [10 nM], final) and recalcification (10 mM, final) to whole blood. Clot contraction was visualized at 10× with digital zoom on a Nikon Eclipse TE2000-U inverted microscope (Nikon Instruments, Melville, NY). Frames depict RBC extrusion from a contracting clot. Black arrowheads highlight a deforming RBC as it exits the clot. Numbers indicate successive frames. Scale bar, 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/16/10.1182_blood-2015-06-652263/4/m_1940f1.jpeg?Expires=1769146610&Signature=j6zECiMc-5EQNww6S8N-WBvAmHbOPYREA003zDZn4HjlJyVLBnuAUK08alaoCnquzwsdG4VrwWyDyNjjx2C49o-D3EjB~Q8JmM-8IgdrWY8Mcdabc-HvBY~sF-PAHcdgGxSoSWQoIi1kVD2gxa3aeLO9FWBCnvYUVB7CZ7UZv~XehBApNuMhCvvuHaEpmC8RyEPxkhk~h8aFwtL0-Wiiq~zU270jUQcvaFww4azj0HSa5bVje8HUCWhTuqNrPMv7UayJ4LPFP4plLKQKcDPIcf7~8ivEX0vHJdPzmey3Denm8oMvA2jZl-I3dGNE2~R2fEbwrAtyGCWQBL~XAu83Xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)