In this issue of Blood, Pereira-Lopes et al demonstrate that a defect in a DNA damage response (DDR) component alters homeostasis of macrophages and their inflammatory responses.1
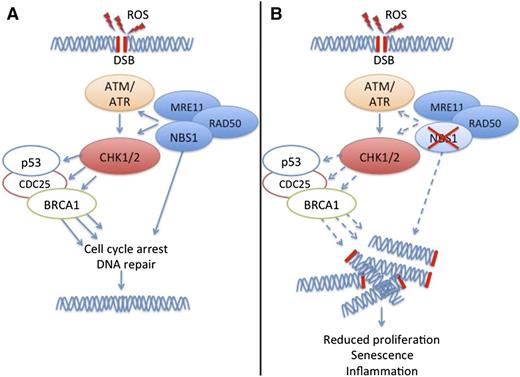
Nbs1 controls macrophage functions. (A) ROS induce DSBs, which trigger DDR involving checkpoint kinases ATM, ATR, CHK1, CHK2, and other checkproteins such as p53, CDC25, and BRCA1. A complex of NBS1, MRE11, and RAD50 facilitates DDR. (B) A deficit of NBS1 impairs DDR, resulting in accumulation of DSBs and subsequent deficit of macrophage proliferation, accelerated macrophage senescence, and excessive secretion of inflammatory cytokines.
Nbs1 controls macrophage functions. (A) ROS induce DSBs, which trigger DDR involving checkpoint kinases ATM, ATR, CHK1, CHK2, and other checkproteins such as p53, CDC25, and BRCA1. A complex of NBS1, MRE11, and RAD50 facilitates DDR. (B) A deficit of NBS1 impairs DDR, resulting in accumulation of DSBs and subsequent deficit of macrophage proliferation, accelerated macrophage senescence, and excessive secretion of inflammatory cytokines.
Cells are constantly exposed to DNA-damaging agents such as ultraviolet light, ionizing radiation, chemotherapeutics, and other genotoxic agents. Double-strand breaks (DSBs), in which both strands in the double helix are severed, are particularly hazardous to the cell because they can lead to genomic rearrangements. Thus, upon sensing DSBs, the cell initiates a DDR that activates DNA repair pathways such as non-homologous end-joining (NHEJ) or homologous recombination.
DDR is controlled by two master kinases, ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3 related (ATR) (see figure). ATM responds to DNA DSBs and disruptions in chromatin structure. ATR primarily responds to stalled replication forks. These kinases phosphorylate downstream signaling molecules such as checkpoint kinase 1 (CHK1) and CHK2 (see figure) leading to checkpoint activation and blockade of cell cycle progression at the G1-S and G2-M boundaries. Checkpoint activation can have several outcomes such as transient delay or sustained arrest of cell cycle progression, initiation of DNA repair, modulation of chromatin remodeling, induction of stress-induced transcription programs, or apoptosis. In addition to ATM, ATR, CHK1, and CHK2, the outcome of checkpoint activation depends on other checkproteins, such as the DNA ends-processing nuclease complex MRE11-RAD50-NBS1 (MRN) (see figure). The MRN complex binds to DSBs and tethers broken ends prior to repair by NHEJ. The MRN complex also participates in activating ATM, CHK1, and CHK2 in response to DNA damage.2
Defects of DDR and NHEJ have been extensively shown to cause genome instability syndromes and cancer. Moreover, NHEJ has been shown to be required for joining DSBs induced during variable diversity joining recombination, the process that generates diversity in B-cell and T-cell receptors in the vertebrate immune system. However, there is emerging evidence that DDR and NHEJ also have an impact on innate immune responses. Several studies have shown that infective agents and microbial products can induce DSBs either directly or indirectly through reactive oxygen species (ROS).3-5 Moreover, unrepaired DSBs activate the cytosolic DNA sensor stimulator of interferon genes, thus inducing the production of interferon alpha and interferon beta, which promote innate immune responses.6-8
Pereira-Lopes et al broaden our knowledge of the physiologic functions of DDR in innate immunity, demonstrating for the first time that multiple macrophage functions are controlled by DDR, in particular, by the Nijmegen breakage syndrome 1 (NBS1) protein, the component of the MRN complex that is responsible for translocation of the MRN complex into the nucleus.9,10 They initially noticed that macrophages stimulated with lipopolysaccharide (LPS) and interferon gamma express high levels of NBS1 protein. To examine the impact of NBS1 on macrophage functions, they examined mice with a hypomorphic mutation of the Nbs1 gene. Macrophages isolated from these mice showed increased DNA damage as measured by phosphorylation of γH2AX under steady-state conditions and after stimulation with LPS, which induces DNA damage by eliciting ROS production (see figure). This defect of NBS1 impaired macrophage proliferation in vitro in response to macrophage colony-stimulating factor and in vivo in response to intraperitoneal injection of zymosan. Macrophage differentiation from bone marrow progenitors was delayed and chromosomal aberrations were increased, although the rate of apoptosis was similar to that of control macrophages. NBS1-deficient macrophages also showed signs of senescence, such as increased levels of p21waf-1, shorter telomeres, and increased secretion of inflammatory cytokines. Finally, NBS1-deficient mice showed exacerbated inflammation in vivo in a model of cutaneous hypersensitivity.
Overall, this insightful study establishes an important function of DDR in limiting immunopathology and facilitating tissue repair during infections and inflammation. Whereas microbial-induced stimuli such as ROS generate DSBs that stimulate DNA sensors and trigger inflammation, DDR negatively regulates this process by reducing the availability of DSBs. This model may help explain the mechanisms underlying some of the clinical manifestations of human NBS, a rare autosomal recessive defect of the NBS1 gene that causes microcephaly, predisposition to cancer, and immunodeficiency.9,10 Moreover, it suggests that the altered equilibrium between DSB generation and DNA repair in macrophages may be an important target for therapeutic intervention in autoimmune diseases such as interferonopathies, which are a result of unwarranted intracellular accumulation of damaged DNA.
Conflict-of-interest disclosure: The author declares no competing financial interests.