Key Points
Prospective analysis of antigen-specific B/T-cell immunity in natural history of human premalignancy.
Stemness antigens and ICPs may be targets for cancer prevention.
Abstract
Blockade of immune checkpoints (ICPs) has led to impressive responses in cancer patients. However, the impact of preexisting immunity and ICPs on the risk of malignant transformation in human preneoplasia has not been prospectively studied. We prospectively analyzed antigen-specific B/T-cell immunity, immune composition of the tumor microenvironment, and the expression of a panel of ICPs on tumor and tumor-infiltrating immune cells in 305 patients with asymptomatic monoclonal gammopathy enrolled in S0120 under the auspices of SWOG. T-cell immunity against stem-cell antigen SOX2 and preserved humoral responses at study entry independently correlated with reduced risk of progression to clinical myeloma. Among the ICPs analyzed, expression of programmed death-ligand 1 (PD-L1) on tumor and infiltrating T cells correlated with increased risk of clinical malignancy, and blockade of this pathway boosted anti-SOX2 immunity in culture. These data suggest that stem-cell antigens and PD-L1 may be targeted for immunoprevention of myeloma. This trial was registered at www.clinicaltrials.gov as #NCT00900263.
Introduction
Blockade of T-cell immune checkpoints (ICPs) leads to tumor regression in several cancers, likely because of reactivation of preexisting tumor immunity.1 In animal models, the immune system can mediate surveillance/editing functions against developing tumors.2 Composition of infiltrating immune cells can impact outcome in established cancers.3 However, the clinical impact of preexisting immunity on the natural history of human premalignancy is not known, as most premalignant lesions are resected upon detection. Multiple myeloma (MM) is preceded by a precursor state termed as monoclonal gammopathy of undetermined significance (MGUS).4 MGUS cells carry most of the genomic changes found in MM.5 Prior studies have shown that the immune system can recognize both MGUS and MM tumor cells.6-8 In these studies, some targets of spontaneous T-cell immunity in MGUS differed from that in MM.9,10 In particular, T-cell immunity against SOX2 was enriched among MGUS patients.9 SOX2 is a core regulator of induced pluripotency and stemness.11 SOX2 is expressed predominantly on the CD138lo subpopulation of tumor cells in MGUS but is essential for clonogenic growth of human MM.9,12,13 Anti-SOX2 T cells efficiently inhibit the clonogenic growth of human MGUS cells.9 MM patients also commonly develop humoral immune paresis. In this study, we have prospectively evaluated a large cohort of patients with precursor asymptomatic monoclonal gammopathies (AMGs) to ascertain the impact of antigen-specific T/B-cell immunity and ICPs on the risk of progression to clinical MM (CMM).
Study design
Patients and trial design
Eligible patients with AMG (both MGUS and asymptomatic MM) were enrolled, following informed consent (in accordance with the Declaration of Helsinki) and approval by an institutional review board, in the prospective clinical trial S0120 conducted under the auspices of the National Clinical Trials Network member SWOG.14 Research blood samples were obtained at initial registration and analyzed for antigen-specific T- and B-cell immunity (supplemental Figure 1; available on the Blood Web site). In some patients, bone marrow specimens were available for analysis of ICPs in tumor and infiltrating immune cells.
Detection of antigen-specific T-cell immunity
T-cell responses against tumor-associated (SOX2) and viral (cytomegalovirus, Epstein-Barr virus, and influenza [CEF]) antigens were analyzed in freshly isolated peripheral blood mononuclear cells as described.9,15 Patients with stimulation index of >2 were scored as positive for antigen-specific T-cell responses.9 T-cell stimulation with mitogen phytohemagglutinin was used as a positive control. For some studies, cells were cultured with anti-programmed death-ligand 1 (PD-L1) antibody (Genentech, CA) concurrent to antigen stimulation.
Detection of antigen-specific B-cell immunity
Levels of clonally uninvolved immunoglobulins were measured as a global measure of humoral immunity. Antibodies against SOX2 were measured as described previously.9 Antibody responses against Epstein-Barr nuclear antigen 1 (EBNA1) and tetanus toxoid were analyzed using an enzyme-linked immunosorbent assay–based method.
Evaluation of immune composition and ICPs
Composition of tumor-infiltrating immune cells (CD4/CD8 T cells, natural killer [NK] cells, CD11b+CD33+ myeloid cells, BDCA1+mDCs, BDCA3+mDCs, and BDCA2+pDCs) and expression of several ICPs (PD-L1/B7H1, B7H3, B7H4, BTLA, Tim3, PD1) in tumor and immune cells were analyzed by multiparameter flow cytometry. In selected cases, expression of ICPs was confirmed by mass cytometry.16
Statistical analysis
Baseline features of patient cohorts were compared using χ2 and Fisher’s exact tests. Cox proportional hazards regression was used to model univariate and multivariate analysis of risk factors.17 Cumulative incidence in the presence of competing risk was used to estimate time to CMM with death as a competing risk.18 Running log rank tests were used to identify optimal splits.19
Results and discussion
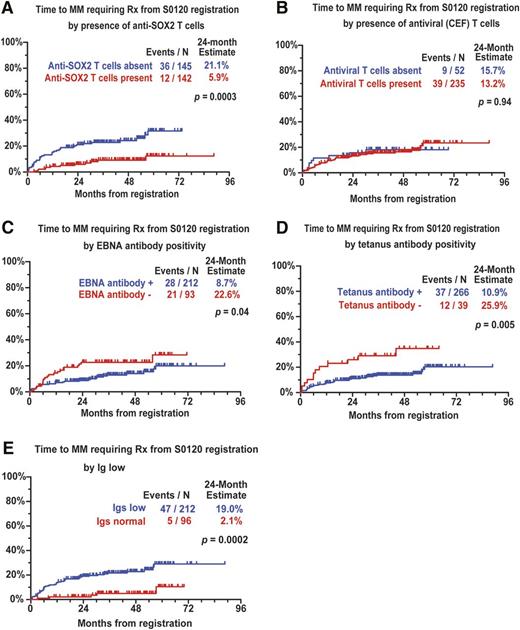
T-cell responses against SOX2 and viral antigens (CEF) were detected in 142 (49%) and 235 (82%) of 287 patients, respectively. Nearly all (286/287) patients had preserved responses to phytohemagglutinin. Patients with anti-SOX2 T cells had less marrow plasmacytosis and lower M spike but did not differ in terms of detection of virus-specific T cells (supplemental Table 1). Anti-SOX2 T cells were detected in 94 of 132 (71%) MGUS and 48 of 155 (31%) asymptomatic multiple myeloma (AMM) patients tested. Reactivity against SOX2-peptide library could be narrowed to single peptides and consisted of both CD4 and CD8+ T cells, consistent with prior studies (data not shown).9 Prior studies have shown that such responses are not detected in fresh peripheral blood mononuclear cells from healthy donors and requires multiple restimulations.9,20 Reduction in uninvolved immunoglobulins was observed in 212 (69%) of 308 patients tested. In spite of this, antibody responses against EBNA1 and tetanus were detected in 212 (70%) and 266 (87%) patients, respectively. In contrast, anti-SOX2 antibodies could be detected in only 30 of 305 patients tested, and their detection was impacted by reduction in uninvolved immunoglobulins. The presence of anti-SOX2 T cells but not virus-specific T cells at baseline was associated with reduced risk of progression to CMM (Figure 1A-B). Preserved levels of uninvolved immunoglobulins and antibody responses against EBNA1 and tetanus also correlated with reduced risk of CMM (Figure 1C-E). Upon multivariate analysis of immune variables, lack of SOX2-specific T cells and reduction in uninvolved immunoglobulins independently predicted increased risk (supplemental Table 2). Importantly, the prognostic impact of SOX2 T-cell immunity was independent of global humoral immune paresis (uninvolved immunoglobulins) and T-cell function (antiviral T cells). Detection of anti-SOX2 T cells strongly correlated with MGUS/AMM clinical classification (supplemental Table 1), and it remained significant in the context of other prognostic factors in a multivariate analysis (supplemental Table 3). Taken together, these data demonstrate an independent impact of anti-SOX2 T cells and global loss of humoral immunity on the risk of malignant transformation in AMG.
Antigen-specific immunity and risk of progression to clinical myeloma. (A) SOX2-specific T cells and risk of progression to clinical myeloma requiring therapy. (B) Virus (CEF)–specific T cells and risk of progression to clinical myeloma requiring therapy. (C) Detection of EBNA1-specific antibodies and risk of progression to clinical myeloma requiring therapy. (D) Detection of tetanus-specific antibodies and risk of progression to clinical myeloma requiring therapy. (E) Reduction in clonally uninvolved immunoglobulins and risk of progression to clinical myeloma requiring therapy.
Antigen-specific immunity and risk of progression to clinical myeloma. (A) SOX2-specific T cells and risk of progression to clinical myeloma requiring therapy. (B) Virus (CEF)–specific T cells and risk of progression to clinical myeloma requiring therapy. (C) Detection of EBNA1-specific antibodies and risk of progression to clinical myeloma requiring therapy. (D) Detection of tetanus-specific antibodies and risk of progression to clinical myeloma requiring therapy. (E) Reduction in clonally uninvolved immunoglobulins and risk of progression to clinical myeloma requiring therapy.
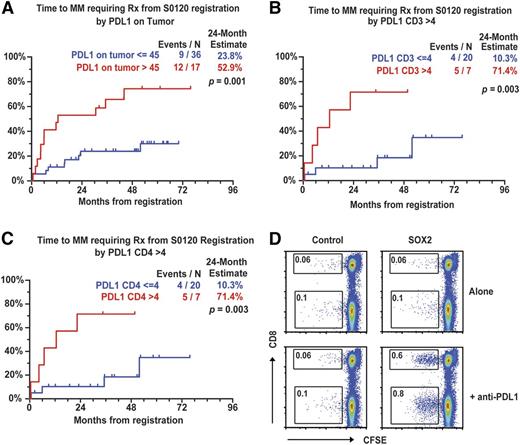
Signaling via coinhibitory signals or ICPs has emerged as a major pathway dampening tumor immunity, and prior studies have demonstrated expression of PD-L1 in human MM driven by inflammatory cytokines.21-23 However, clinical significance of these pathways in premalignancy is not known, with most analyses restricted to single markers and cell types. Both MGUS and AMM plasma cells (PCs) were found to express PD-L1/B7-H1 and/or B7H3, but not B7H4 (supplemental Figure 2A and supplemental Table 4). PD-L1 was also expressed on CD14+ myeloid cells and, to a lesser extent, on T cells, NK cells, BDCA1+mDCs, but not BDCA3+DCs and pDCs (supplemental Figure 2B-C and supplemental Table 4). PD-L1 expression on PCs was also confirmed by mass cytometry and did not differ between PCs expressing clonal or nonclonal immunoglobulins (supplemental Figure 3). Among other ICPs, tumor-infiltrating T cells also expressed BTLA, whereas Tim-3 was primarily expressed on NK cells (supplemental Figure 2C and supplemental Table 4). PD-L1 expression was higher in AMM than in MGUS PCs (supplemental Table 4). These cohorts did not differ in terms of other ICPs or proportion of infiltrating immune cells (supplemental Table 4). The cohort with PD-L1high tumors also had higher M spike and marrow plasmacytosis (supplemental Table 5). Expression of PD-L1 on tumor cells (>45%, hazard ratio = 3.8, P = .001) and CD3+ T cells (>4%, hazard ratio = 6.8, P = .003), and specifically CD4+ T cells, but not myeloid cells, correlated with an increased risk of progression to CMM (Figure 2A-C). Expression of PD-1 or BTLA on T cells or Tim-3 on NK cells was not predictive of risk (supplemental Table 6). The presence of SOX2-specific T cells correlated inversely with the presence of PD-L1, particularly on T cells (supplemental Table 7). In order to test the possibility that PD-L1-mediated signaling may contribute to suppression of anti-SOX2 T cells,24 we analyzed whether antibody-mediated blockade of PD-L1 can enhance SOX2-specific T cells in culture. PD-L1 blockade led to an increase in antigen-dependent proliferation of SOX2-specific T cells in 4 of 6 AMM patients tested (Figure 2D). Together, these data demonstrate that the expression of PD-L1 on both tumor cells and T cells is associated with increased risk of malignancy. The association between SOX2 immunity and PD-L1, as well as PD-L1 as a risk factor, is a preliminary finding and warrants further investigation.
ICPs and risk of progression to clinical myeloma. (A) Expression of PD-L1 on tumor cells and risk of progression to CMM requiring therapy. (B) Expression of PD-L1 on CD3+ T cells and risk of progression to clinical myeloma requiring therapy. (C) Expression of PD-L1 on CD4+ T cells and risk of progression to clinical myeloma requiring therapy. (D) Effect of PD-L1 blockade on antigen-dependent proliferation of SOX2-specific T cells in culture.
ICPs and risk of progression to clinical myeloma. (A) Expression of PD-L1 on tumor cells and risk of progression to CMM requiring therapy. (B) Expression of PD-L1 on CD3+ T cells and risk of progression to clinical myeloma requiring therapy. (C) Expression of PD-L1 on CD4+ T cells and risk of progression to clinical myeloma requiring therapy. (D) Effect of PD-L1 blockade on antigen-dependent proliferation of SOX2-specific T cells in culture.
To our knowledge, these are the first data to prospectively evaluate the clinical impact of antigen-specific B/T-cell immunity and ICPs in a human premalignancy. The strengths of this study are its prospective nature with uniform follow-up of patients and large sample size. Because of the limits of sample availability, analysis of antigen-specific T cells was limited to circulating T cells. However, we have previously demonstrated tumor-specific T cells to be enriched in the tumor bed.6,7,9 The finding that T-cell immunity to a single antigen correlates with outcome is surprising as immunity against a growing tumor is likely to be directed against mutation-derived neo-epitopes.2,7 One possible explanation is that stemness pathways targeted by this immune response may be important in myelomagenesis.12,13,25 This is also supported by prior studies showing that SOX2-specific T cells and SOX2-RNA interference can reduce clonogenic growth of MM.9,12 Preexisting SOX2 immunity also correlated with clinical response to checkpoint blockade in lung cancer.15 Together, these data suggest that both ICPs and stemness antigens may be important for novel approaches to prevent CMM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Public Health Service/Department of Health and Human Services grants awarded by the National Institutes of Health National Cancer Institute (NCI) National Clinical Trials Network (CA1800888, CA1800819, CA180801, CA180826, CA180846, CA180830, and CA180858) and the NCI Community Oncology Research Program grants (CA189853 and CA189953). M.V.D. is supported in part by NCI grants (A106802 and CA135110).
Authorship
Contribution: M.V.D. designed research, served as principal investigator for the study, analyzed data, and wrote the manuscript; R. Sexton performed statistical analysis; K.M.D. performed and supervised analysis of immune data; R.D., L.Z., R. Sundaram, and S.S. performed some of the assays and analyzed data; J.J.C. supervised statistical analysis; R.Z.O. is the current chair of the SWOG myeloma committee; and B.B., prior chair of the SWOG myeloma committee, served as coprincipal investigator for the study.
Conflict-of-interest disclosure: S.S. is currently employed by Bristol Myer Squibb (Princeton, NJ). The remaining authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, Yale University, 333 Cedar St, New Haven, CT 06510; e-mail: madhav.dhodapkar@yale.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal