To the editor:
Major advances have been made in the treatment of patients with acute promyelocytic leukemia (APL) with the development of molecularly targeted therapies, first with the introduction of all-trans retinoic acid (ATRA) added to standard chemotherapy and more recently with the advent of arsenic trioxide (ATO).1 Recent data from a pivotal phase 3 trial comparing standard ATRA plus chemotherapy vs ATRA-ATO2 and subsequent follow-up data strongly suggest that patients with low- to intermediate-risk disease may be cured by targeted treatment alone and without chemotherapy.3
However, the important progress made in the understanding of APL biology and the excellent clinical results have not been paralleled by a full understanding of the impact of the disease and its treatment on patients’ lives. APL is a life-threatening condition and a medical emergency. Based on international guidelines, immediate start of treatment with ATRA is recommended even before the diagnosis is fully confirmed by molecular analysis.4 Therefore, it is self-evident that major efforts have historically been devoted to saving the lives of these patients and improving treatment outcomes.
It is now time to also better understand how APL treatments affect patients’ health-related quality of life (HRQOL) and how we can best help these patients to fully recover from the negative consequences of therapies. What are the main functional limitations or specific symptoms that clinicians should mostly pay attention to? This type of information, for example, could help to facilitate APL tailored-based supportive care programs.
Although 3 recent APL randomized controlled trials have included patient-reported HRQOL as a formal end point of the study,5-7 much remains to be learned about HRQOL. Typically, APL patients receive high-intensity and long-lasting therapies (ie, induction, consolidation, and maintenance therapy).
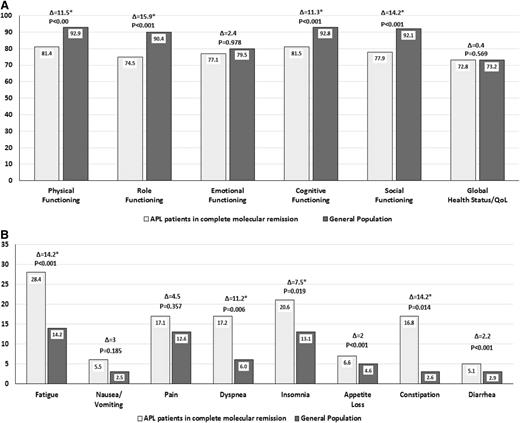
An accepted surrogate of treatment efficacy and favorable outcome in the treatment of this disease is the achievement of complete molecular remission. We have previously shown that all patients included in our randomized controlled trial achieved complete molecular remission at the end of the third consolidation cycle2 and that, at this time, no major differences existed in HRQOL outcomes between patients randomized to ATRA-ATO and those randomized to ATRA plus chemotherapy.5 We herein examine to what extent these responding patients (after the third consolidation cycle) (N = 119), treated with either ATRA-ATO or ATRA plus chemotherapy, might resume their “normal” HRQOL (predisease) by comparing their profile with that of their peers in the general population.8 Sex- and age-adjusted comparisons were made with the EORTC QLQ-C30 questionnaire,9 and clinically relevant thresholds for each specific scale were determined using previously published criteria.10 Results are depicted in Figure 1.
Health-related quality of life of APL patients in complete molecular remission compared with general population. (A) Self-reported functional scales and global quality of life of APL patients in molecular complete remission compared with general population norms adjusted by age and gender. (B) Self-reported symptoms of APL patients in molecular complete remission compared with general population norms adjusted by age and gender. For functional and global quality of life (QoL) scales, higher scores indicate better outcomes; for symptom scales, higher scores indicate higher symptom severity. Analysis is based on 119 patients (61 ATRA plus ATO patients and 58 ATRA plus chemotherapy patients). Asterisk indicates at least a small clinically relevant difference.10
Health-related quality of life of APL patients in complete molecular remission compared with general population. (A) Self-reported functional scales and global quality of life of APL patients in molecular complete remission compared with general population norms adjusted by age and gender. (B) Self-reported symptoms of APL patients in molecular complete remission compared with general population norms adjusted by age and gender. For functional and global quality of life (QoL) scales, higher scores indicate better outcomes; for symptom scales, higher scores indicate higher symptom severity. Analysis is based on 119 patients (61 ATRA plus ATO patients and 58 ATRA plus chemotherapy patients). Asterisk indicates at least a small clinically relevant difference.10
Statistically and clinically meaningful impairments in physical (Δ = 11.5), role (Δ = 15.9), social (Δ = 14.2), and cognitive functioning (Δ = 11.3) were found for APL patients compared with general population norms (Figure 1A). With regard to symptoms, the 3 major clinically relevant impairments in APL patients were found for fatigue (Δ = 14.2), constipation (Δ = 14.2), and dyspnea (Δ=11.2) (Figure 1B).
Our results suggest that, although fully responding to therapy, patients’ HRQOL is still compromised in several respects. Importantly, the burden of symptoms was sustained despite the rather younger age of these patients (median age of 45 years). This might reflect the heavy burden of aggressive therapies and related side effects such as cytopenias, mucositis, and infections for those treated with ATRA plus chemotherapy and liver toxicity for those treated with ATRA-ATO. Long-term HRQOL studies in these patients are urgently needed to further investigate at which point in time, during the course of current APL treatments, patients can expect to fully recover.
Authorship
Acknowledgments: The authors thank all patients who participated in this study and all participants and research staff of all centers within the Gruppo Italiano Malattie Ematologiche dell’Adulto, the German-Austrian Acute Myeloid Leukemia Study Group, and Study Alliance Leukemia.
Contribution: F.E. and F.L.C. conceived and designed the study; F.C. and F.E. analyzed statistics; and F.E., F.M., U.P., F.C., and F.L.C. wrote the manuscript, analyzed and interpreted data, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Efficace, Health Outcomes Research Unit, Italian Group for Adult Hematologic Diseases (GIMEMA), GIMEMA Data Center, Via Benevento, 6, 00161 Rome, Italy; e-mail: f.efficace@gimema.it.