Abstract
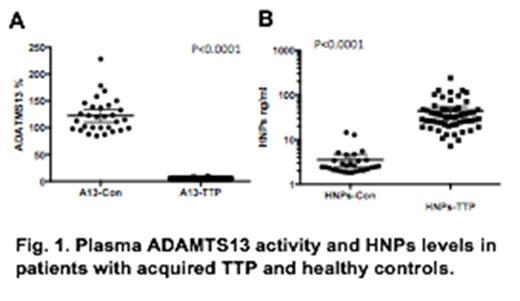
Acquired thrombotic thrombocytopenic purpura (TTP) is a potentially fatal syndrome, resulting from autoantibodies against the metalloprotease ADAMTS13. Autoantibodies bind and inhibit plasma ADAMTS13 activity, leading to exaggerated platelet agglutination and thrombus formation in small arteries and capillaries. However, plasma ADAMTS13 deficiency or the presence of anti-ADAMTS13 autoantibodies is not sufficient to cause the disease. Infection or inflammation often precedes the acute onset of TTP. We hypothesize that neutrophil activation or complement activation vial alternative pathway may be the trigger for TTP. In this study, plasma samples were collected prior to plasma exchange from 58 adult patients with acute TTP between 2006 and 2015 at the University of Alabama at Birmingham Medical Center. All patients were diagnosed having TTP on the basis of severe thrombocytopenia, microangiopathic hemolytic anemia, and severe deficiency of plasma ADAMTS13 activity (less than 10%) and the presence of ADAMTS13 inhibitors. All patients were treated by plasma exchange. Thirty plasma samples from healthy individuals were collected for controls. Plasma levels of human neutrophil peptides (HNP-1, -2, and -3) released from activated neutrophils and alternative pathway complement activation markers (iC3b, C4d, C5b-9, and Bb) were determined by enzyme-linked immunosorbent assays (ELISAs). We showed that plasma levels of HNP-1, -2, and -3 in TTP patients were elevated on average by 10-fold when compared with those in the healthy controls (44.12 ± 39.53 ng/ml vs. 3.54 ± 2.97 ng/ml, means ± SD) (p <0.0001). Whereas plasma levels of Bb and iC3b were only modestly increased in TTP patients (2.94 ± 1.82 µg/ml vs. 1.39 ± 0.34 µg/ml for Bb, p <0.0001, and 16.21 ± 9.71 µg/ml vs. 12.13 ± 3.07 µg/ml for iC3b, p =0.028). No significant difference was detected in the plasma levels of C4d and C5b-9 between TTP patients and healthy controls (p >0.05). To differentiate HNP-1 from HNP-2 or HNP-3, we developed a LC/MS mass spectrometric assay and showed that all subtypes of HNPs in TTP patients were significantly elevated when compared with those in the healthy controls (p<0.001). In general, there was good concordance between ELISA and LC/MS MS in the measurement of all three HNPs. These results demonstrate that innate immunity (i.e. activation of neutrophils) and to a lesser degree the activation of complements via alternative pathway may play a role in pathogenesis of TTP in light of severe ADAMTS13 deficiency. Our findings provide rationales for developing novel targeted therapies for acquired TTP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal