Abstract
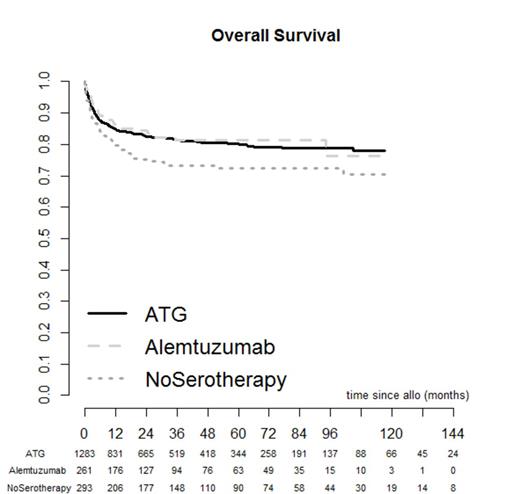
To determine the optimal serotherapy regimen in idiopathic aplastic anaemia stem cell transplantation, we analysed 1837 patients who underwent either in vivo T cell depletion with either ATG (n=1283) or Alemtuzumab (n=261) or no serotherapy (n=293) as part of their conditioning regimen. All patients had either undergone a matched sibling or matched unrelated donor stem cell transplant (at least 6 out of 6). Data was obtained retrospectively from the EBMT SAA database, between the periods 2000-2013. The major endpoints were graft versus host disease, overall survival and event free survival. Events were classified as graft failure, secondary malignancy, relapse and requirement for second transplant. The median follow up was 34 months in the ATG group, 30.9 months in the Alemtuzumab group and 47.9 months in the no serotherapy group. Rate of grade 2-4 acute GVHD was 19.1% without serotherapy; this was higher than that observed with both ATG (13.3%, p<0.001) and Alemtuzumab (6.7%, p<0.001; ATG vs Alemtuzumab: p=0.012). Cumulative incidence of chronic GVHD at 36 months was 30.4% without serotherapy; this was higher than that observed with both ATG (20.8%, p=0.021) and Alemtuzumab (14.7%, p=0.003; ATG vs Alemtuzumab: p=0.083). In multivariate analysis, grade 2-4 acute and chronic GVHD rates were significantly lower with Alemtuzumab compared to no serotherapy (Odds Ratio ratio (OR) = 0.16, p< 0.001 95%CI 0.08-0.31 and HR= 0.38, 95% CI 0.24-0.62, p< 0.001 respectively). Similarly, acute and chronic GVHD were lower with Alemtuzumab compared to ATG (OR = 0.26;p<0.001 95%CI 0.14-0.47 and HR = 0.58; 95%CI 0.38-0.89, p=0.012 , respectively). Acute and chronic GVHD were higher without serotherapy compared to ATG (OR = 1.65; p =0.01 95%CI 1.12-2.41 and HR = 1.51; 95%CI 1.12-2.04, p=0.008 , respectively). There was no difference in event free survival between the three groups. However, overall survival at 36 months was 73.3%, 81.3% and 81.5 in no serotherapy, ATG and alemtuzumab, respectively (ATG vs no serotherapy p=0.01; Alemtuzumab vs no serotherapy p=0.025; Alentuzumab vs ATG p=0.604). In multivariate analysis overall survival favoured in vivo T cell depletion compared to no serotherapy; (Alemtuzumab vs no serotherapy, HR=0.44; 95%CI 0.29-0.67, p<0.001); (no serotherapy vs. ATG HR=1.55; 95%CI 1.19-2.01, p=0.001). Among serotherapy, Alemtuzumab was associated with better OS as compared with ATG (OR=0.68; 95%CI 0.48-0.98, p=0.037). Our results suggest that use of in vivo T cell depletion in sibling and unrelated matched stem cell transplantation in idiopathic aplastic anaemia leads to a survival advantage. Alemtuzumab is associated with less GVHD than ATG without affecting event free survival.
Snowden:Celgene: Other: Educational support, Speakers Bureau; Janssen: Other: Educational support, Speakers Bureau; MSD: Consultancy, Other: Educational support, Speakers Bureau; Sanofi: Consultancy. Dufour:Pfizer: Consultancy. Risitano:Pfizer: Consultancy; Novartis: Research Funding; Rapharma: Consultancy, Research Funding; Alnylam: Consultancy, Research Funding; Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Off Label Use: this paper includes discussion of the use of alentuzumab for GVHD prophylaxis, which is currently off-label.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal