Abstract
Introduction: Acute myeloid leukemia (AML) is the most common blood cancer in adults and the second most common in children. Despite intensification of chemotherapeutic regimens, survival rates have plateaued. AML is therefore a candidate for novel therapeutics, specifically immune checkpoint inhibitors (ICIs). ICIs have shown significant promise in solid malignancies and more recently in Hodgkin's disease. However, it is currently not possible to predict which diseases and/or patients will benefit from ICIs. We are therefore, performing detailed studies to characterize the phenotype such that patients can be matched with ICI regimens to which they are most likely to benefit. Specifically, we are identifying the relevant lymphoid cell subsets present in the marrow of patients with AML, evaluating their differentiation and activation status, and quantifying the density of immune modifiers on cells of interest. We are also performing functional assays to further define the status of T cells in the AML microenvironment by evaluating the responsiveness of these cells to stimulation. Additionally, these studies assess the impact of ICI on the proliferative and cytokine production capacity of T cells and tumor cells resident in AML bone marrow.
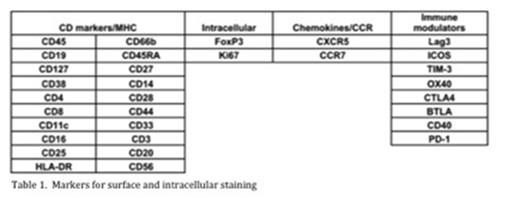
Methods: Immunophenotyping was performed on mononuclear cells from primary AML patient specimens using mass cytometry by staining samples with a panel containing 32 antibodies to surface markers and cytokines (Table 1). Each antibody was tagged with a unique lanthanide isotope and data was obtained using a DVS time-of-flight mass cytometer (CyTOF). T cells were defined by CD45 and CD3 positivity and then further differentiated based on the presence of CD4 or CD8. CD4 and CD8 cells were further classified into naive (CCR7+, CD45RA+), central memory (CCR7+, CD45RA-), effector memory (CCR7-, CD45RA-), and effector memory CD45RA+ (TEMRA)(CCR7-, CD45RA+) cells. Functional assays measured both the proliferative capacity and cytokine production profile of bone marrow T cells in response to CD3 stimulation in the context of the tumor microenvironment. Changes to the baseline T cell functional profile in the presence of various ICIs, including antibodies against PD1, CTLA4, TIM3, and VISTA, were measured.
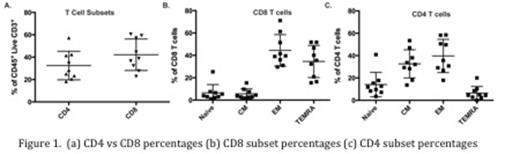
Results: Bone marrow samples from 9 patients with AML were collected. T cell percentages ranged from 0.2% to 4.3% with an average CD4:CD8 ratio of 0.39 to 2.0 (Figure 1a). Within the CD8 T cell population, naive, central memory, effector memory and TEMRA cells ranged from 1.1% to 25.4%, 1.8% to 9.7%, 15.5% to 51.8% and 15.5% to 51.8%, respectively (Figure 1b). Within the CD4 T cell population, naive, central memory, effector memory T cells and TEMRA cells ranged from 3.5% to 40.9%, 13.6% to 53.4%, 17.9% to 58.5%, and 0% to 10.2% respectively (Figure 1c). T cell expression of immune modulators varied (Figure 2) and there were differential effects of several ICIs on T cell proliferation and production of cytokines, including interferon gamma, interleukin-6, and tumor necrosis factor-alpha.
Conclusion: These results provide the beginning of a functional and phenotypic description of the immune status in the marrow of patients with AML. Our results show that, like the disease itself, the immune microenvironment is very heterogeneous between samples with differential responses to ICIs suggesting that certain subsets of AML may be potential candidates for immunotherapy. This is a report of initial findings from our study; accrual of patient samples is ongoing to expand the power of our observations.
Huang:Janssen Pharmaceuticals: Employment. Sasser:Janssen Pharmaceuticals: Employment. Tyner:Aptose Biosciences: Research Funding; Array Biopharma: Research Funding; Constellation Pharmaceuticals: Research Funding; Janssen Pharmaceuticals: Research Funding; Incyte: Research Funding. Lind:Janssen Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal