Abstract
Background: MYD88 mutations are present in over 95% of patients with Waldenstrom's Macroglobulinemia (WM), and promote Myddosome self-assembly that triggers NFκB-dependent survival through BTK and IRAK1/IRAK4 (Blood 122(7):1222-32). While current therapeutic strategies are aimed at blocking these downstream kinases, peptidomimetics that interfere with Myddosome self-assembly may offer a more targeted approach for blocking aberrant MYD88 signaling.
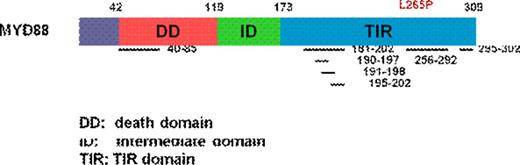
Methods: We expressed by lentiviral transduction mini-peptides of MYD88 Toll/Interleukin-1 Receptor (TIR) or Death Domain (DD) sequences in mutated MYD88 WM and wild-type MYD88 control cells (Figure 1). We used phospho-flow analysis to evaluate for changes in pBTK, pIRAK1/IRAK4, and pNFKB, and determined cell growth and survival by Alamar Blue Assay, Annexin V, and cleaved Caspase 3 staining.
Results: Transduction of TIR or DD mini-peptides in mutated MYD88 WM cells but not wild-type MYD88 control cells reduced NFKB activation and tumor cell growth, and prompted Annexin V and/or cleaved Caspase 3 staining. TIR interfering mini-peptides impacted BTK but not IRAK1/IRAK4 activation, whereas DD interfering mini-peptides showed an opposite effect.
Conclusions: The findings demonstrate differences in BTK versus IRAK1/IRAK4 directed NFKB signaling in response to Myddosome self-assembly in MYD88 mutated WM cells. The feasibility of directly targeting MYD88 homodimerization to block aberrant MYD88 signaling was also recognized, and suitable peptide sequences for the development of peptidomimetics that interfere with Myddosome self-assembly and signaling were identified. The findings provide a framework for direct targeting of the Myddosome in MYD88 mutated WM disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal