Abstract
Introduction: Novel substances such as the next generation IMiD pomalidomide or the recently approved next generation proteasome inhibitor carfilzomib (Cfz) have considerably expanded our treatment options in MM, both of them influencing multiple myeloma (MM) interaction with bone marrow stroma cells (BMSCs), that provides an interesting target for anti MM therapy. More compounds directed at this disease critical crosstalk are currently under investigation, however the development of novel drugs remains inefficient, displayed by a substantial drop out rate of the 376 preclinical single agents tested since 1961 (Rongvaux, Annu Rev Immunol. 2013; Schüler, Expert Opin Biol Ther. 2013; Kortüm, CLML 2014). Our focus in the projected presented here was to develop a novel bone-derived in vitro 3D co-culture model specifically adapted to mimic the BM niche to more closely study the role of bone and BM bystander cells and to perform more reliable ex vivo compound screening in MM.
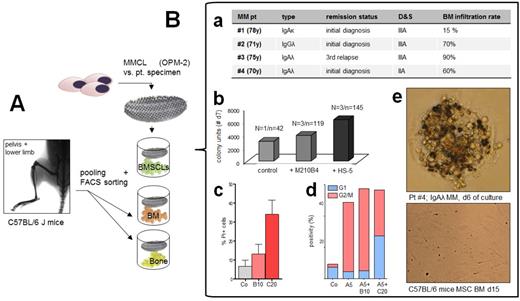
Methods: Previous 2D models were compared to a novel 3D co-culture model (agarose matrix interlayer, 100 microwells/cm², 1.5mm in depth, permeable for oxygen+cytokines, but not for BMSCs utilizing U266, RPMI-8226, OPM-2 and primary BM patient (pt) cells, with and without HS-5 vs. M210B4 stroma support (Fig. A + Fig. B.a. for pt characteristics). Analyses covered Trypan Blue, Annexin/PI, MTT, FACS, cell cycle analyses and H2B-mCherry/cytochrome c-GFP assays (Udi, Br J Hematol. 2013). In a next step, primary bone-derived stroma cells were acquired from bones of C57BL/6 J mice. Bones were flushed, digested and FACS sorted in order to acquire single BM and bone bystander cell subtypes (MSPCs [mesenchymal stem and progenitor cells], endothelial cells, osteoblasts, PAS [PDFGRalphaSca1] and CaRs [CXCL12-abundant reticular cells]) which were then compared to HS-5 and M210B4 with regard to growth support, cytokine secretion and protection from anti-MM substances.
Results: MMCLs and pt specimens were cultured at different concentrations (10 vs. 100 cells per microwell) with and without M210B4 demonstrating a growth advantage with vs. without M210B4 (Fig. B.b). Liquid overlay technique allowed cluster formation of pt specimens leading to more reliable propagation of pt material for up to 20d of culture. Apoptotic changes were assessed by confocal microscopy of RPMI8226 co-expressing fluorescently labelled histone 2B-mCherry (red) and cytochrome c-GFP (green) as indicators of late and early apoptosis. Comparing BMSCLs with regard to their MM growth support capacities, human HS-5 proved even more beneficent than M210B4 stroma (Fig. B.b). Phenotypic analyses of pt specimens showed decreased CXCR4 expression with vs. without BMSCs suggesting a dynamic regulation of homing molecules. The model was then used as an ex-vivo platform allowing both cytotoxicity and cell cycle analyses for the combination of bortezomib (Btz) vs. Cfz with ARRY-520 (kinesin spindle protein inhibitor). Btz (10nM) and Cfz (20nM) proved significantly cytotoxic compared to the control (U266 and pt specimens, respectively) after 48h of single agent treatment Fig.B.c). Compared to Btz (10nM, B10) and Cfz (20nM, C20) as single agents, the additional combination with ARRY-520 showed stronger additive cytotoxicity for Btz (A5+B10, median: 37.5% vs. 13.1%) than for Cfz (A5+C20, 38.6% vs. 34.1%). To note, the model could also be utilized for more profound analyses as depicted for G2/M cell cycle studies in Fig.B.d. 5nM ARRY-520 (A5) led to significant accumulation of OPM-2 cells in G2/M arrest after 48h of treatment confirming prior analyses (Hernández-Garcia Blood Suppl 4710,2014; Fig. B.d). Co-culture studies with different subsets of BM and bone-derived bystander cells are currently ongoing and will be presented at the meeting (Fig. B.e).
Conclusions: More complex, 3D bone-derived high-throughput in vitro models are urgently needed to better predict the potency of preclinically tested agents and to better estimate the likelihood of their later clinical adoption into phase I-II trials. With this work, we provide an innovative model which reflects the BM microenvironment as a crucial predictor for in-vivo sensitivity as shown for ARRY-5200. This ex-vivo approach helps to better incorporate MM growth support by bone and BM-derived bystander cells and thus depicts a valid tool to better characterize the role of the BM niche in myeloma.
Engelhardt:Deutsch Krebshilfe: Other: grant. Wäsch:German Cancer Aid: Research Funding; Comprehensiv Cancer Center Freiburg: Research Funding; Janssen-Cilag: Research Funding; MSD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal