Abstract
Introduction
Up to 50% of patients with acute GVHD (aGVHD) have a suboptimal response to first line therapy with corticosteroids. Extracorporeal photopheresis (ECP) is one of the recommended second line options. Overall response rates of 60-77% have been reported with ECP as second line therapy for aGVHD. Factors associated with lower response and survival were Grade >II at onset and number of organs involved. In a large retrospective study of ECP as second line therapy, 19.5% of patients developed chronic GVHD (cGVHD). Previous studies have included small numbers of patients with aGVHD post T cell depleted transplants or Donor Lymphocyte Infusion (DLI).
Methods
We conducted a retrospective analysis of outcomes in 40 patients treated with ECP for steroid refractory or steroid dependent aGVHD at 2 UK transplant centres. The aim was to analyse overall survival and long-term outcomes. All patients received 2-3 treatments per week for 8 weeks after which schedules differed according to centre, cessation or gradual taper.
Results
Between 2006 and 2014, 44 patients had received ECP as second line therapy, 4 were excluded from analysis as they had received less than 4 treatments. The median age was 51years (21-67years). Table 1 illustrates details of conditioning and GVHD prophylaxis. Thirty percent of patients were post DLI: 5 (12.5%) for disease relapse and 7 (17.5%) for mixed chimerism. Transplant conditioning included T cell depletion in 24 (60%): alemtuzumab n=17, ATG n=7. The median duration of steroids pre-starting ECP was 18 days (range 5-56). Using Modified Glucksberg crtieria at the onset of ECP, 5 patients (12.5%) had Grade II, 30 (75%) Grade III (including 6 with stage 4 gut) and 5 (12.5%) Grade IV aGVHD. Nine patients (22.5%) had 1 organ involvement and 31 (77.5%) had 2 or more organs involved. The dominant organ was gut in 21 (52.5%), liver in 10 (25%)and skin in 9 (22.5%). Maximal response rates were CR 57.5%, PR 27.5% and NR 15%. A number of patients received additional immunosuppressive therapy, predominantly anti-TNF antibodies: 14 patients (35%) (etanercept n=11, infliximab n=3).
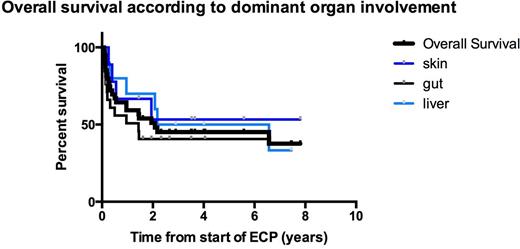
Using Kaplan-Meier analysis, censoring at last follow up, the overall survival from start of ECP at 5 years was 45.1% (95% CI 31.85-63.04%) Figure 1. The median survival was 2 years (27days-7.8yrs). In the DLI group, the 5 year overall survival was 59.8%. In a subgroup log rank Mantel-Cox analysis no significant difference was observed in the 5 year overall survival according to dominant organ involved: 53% skin, 40.6% gut and 50% liver (p=0.77) Figure 2.
The cumulative incidence of death was 57.5% (23patients). The leading cause of death was infection 13/23 deaths (56.5%) followed by 5 deaths due to GVHD, 4 due to relapse, 1 from unrelated causes.
The incidence of chronic GVHD was 55%. Treatment for cGVHD varied with 8 patients continuing ECP >12 weeks on a tapering schedule, 2 patients restarting ECP for cGVHD and the others receiving a variety of therapies including MMF, tacrolimus, Rituximab and imatinib. At last follow up, 17 patients were alive, 9 were off immunosuppression (2 of whom had not developed cGVHD) and 8 were still on immunosuppression. The performance status in the 17 surviving patients was ECOG 0= 8, 1=8 and 2=1.
Discussion
Our study shows an encouraging 5 year overall survival of 45.1% in a cohort with predominantly severe Grade III aGVHD. The 5 year survival was higher in the DLI group at 59.8%. Fifty three percent had stage 3-4 gut involvement, which is a recognised poor risk feature. Infection was the leading cause of death, which is of particular relevance in the T deplete setting. Whilst 55% developed cGVHD, over a long follow up period (median of 4years), the cGVHD resolved and performance status was preserved in most of the surviving patients. The incidence of cGVHD was higher than in other studies but the patients had higher grade of aGVHD at onset, were older and had multi-organ involvement. These findings need to be confirmed in prospective studies.
| Pretransplant Characteristics . | No. of patients (%) . |
|---|---|
| Conditioning Regimes | |
| Myeloablative | 10 (25%) |
| Non-myeloablative | 18 (45%) |
| Total post DLI | 12 (30%) |
| T cell depletion | 24 (60%) |
| Alemtuzumab | 17 (42.5%) |
| ATG | 7 (17.5%) |
| GVHD prophylaxis | |
| CsA | 20 (50%) |
| CsA/MTX | 9 (22.5%) |
| CsA/MMF Tacro/MMF | 8 (20%) 3 (7.5%) |
| Donor Source | |
| Matched sibling Matched unrelated donor 10/10 9/10 8/10 Double Cord | 9 (22.5%) 21 (52.5%) 3 (7.5%) 1 (2.5%) 6 (15%) |
| Pretransplant Characteristics . | No. of patients (%) . |
|---|---|
| Conditioning Regimes | |
| Myeloablative | 10 (25%) |
| Non-myeloablative | 18 (45%) |
| Total post DLI | 12 (30%) |
| T cell depletion | 24 (60%) |
| Alemtuzumab | 17 (42.5%) |
| ATG | 7 (17.5%) |
| GVHD prophylaxis | |
| CsA | 20 (50%) |
| CsA/MTX | 9 (22.5%) |
| CsA/MMF Tacro/MMF | 8 (20%) 3 (7.5%) |
| Donor Source | |
| Matched sibling Matched unrelated donor 10/10 9/10 8/10 Double Cord | 9 (22.5%) 21 (52.5%) 3 (7.5%) 1 (2.5%) 6 (15%) |
Radia:Therakos: Other: ASH travel expense, Speakers Bureau. Off Label Use: Extracorporeal Photopheresis (ECP) is not licensed for use in GVHD but is recommended in international guidelines as a therapeutic option.. McLornan:Novartis: Research Funding, Speakers Bureau. Russell:Therakos: Other: shares.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal