Abstract
Background: Children with acute lymphoblastic leukemia (ALL) receive multiple doses of intrathecal methotrexate during treatment. Methotrexate is a folate analogue that competitively inhibits dihydrofolate reductase leading to a decrease in purine and pyrimidine synthesis. The administration of methotrexate also leads to alterations of the methyl transfer pathway that may be determinants of toxicity and therapeutic response.
Objective: To describe the changes of the methyl transfer pathway in CSF seen with sequential administration of intrathecal methotrexate in children receiving therapy for ALL.
Methods: Children with ALL received age-based doses of intrathecal methotrexate on days 8, 29, 36, 43 and 50 of treatment. No oral or intravenous methotrexate was administered during the first 50 days of treatment. CSF and plasma samples were collected at the time of each intrathecal therapy. Concentration of 5-methyltetrahydrofolate (5-MTHF), 5,10-methylenetetrahydrofolate (5,10-MeTHF), betaine and choline were measured using reversed phase LC-MS/MS.1 Control samples were collected from patients with AML who were not exposed to any methotrexate.
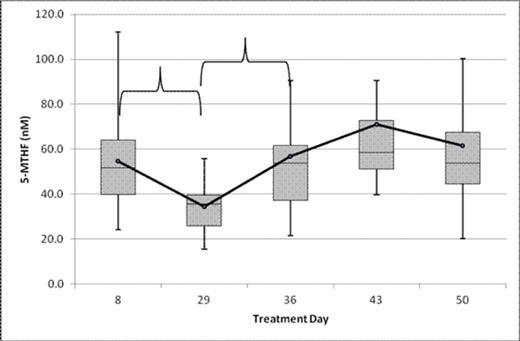
Results: 39 children with ALL and 7 with AML were included and contributed samples to our analysis. The mean baseline CSF 5-MTHF concentration (treatment day 8) measured in the ALL patients was 54.8 nM. The concentration of CSF 5-MTHF decreased to 34.4 nM (p<0.001) on treatment day 29, 21 days after receiving a single dose of intrathecal methotrexate. On treatment day 36, the CSF 5-MTHF was 56.7 nM, indicating a return to baseline levels. Despite repeated weekly administration, the concentration on treatment days 43 and 50 did not significantly change (Figure 1).
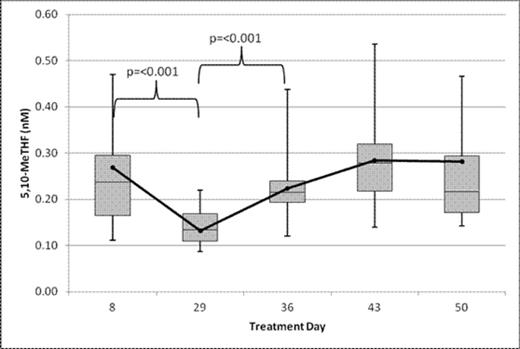
Similar results were seen when CSF concentrations of 5,10-MeTHF were analyzed. The baseline mean 5,10-MeTHF concentration was 0.27 nM on treatment day 8 and this decreased to 0.13 nM 21 days later (p<0.001). By treatment day 36, mean CSF levels of 5,10-MeTHF were near baseline at 0.22 nM and no further significant changes in levels were seen on treatment days 43 or 50 (Figure 2).
Day of Treatment
The mean RBC 5-MTHF concentration was 641.9 nM on treatment day 8, 611.9 nM on day 29, 887.2 nM on day 36, 1197.3 nM on day 43 and 1063.3 nM on day 50. The mean RBC 5-MTHF on treatment days 43 and 50 was significantly increased when compared to baseline levels (p<0.001).
As CSF folate is depleted and replenished during the first two months of treatment, other biochemical alterations of the methyl transfer pathway with the CSF were evaluated. Levels of CSF betaine steadily increased with ongoing intrathecal treatment while CSF choline did not change (Table 1).
Changes in Folate Homeostasis During Therapy for ALL Through Treatment Day 50
| . | Control . | Day 8 . | Day 29 . | Day 36 . | Day 43 . | Day 50 . | p . |
|---|---|---|---|---|---|---|---|
| CSF 5-MTHF (nM) | 84.6 | 54.8 | 34.0 | 52.3 | 62.2 | 55.0 | <0.001 |
| CSF 5,10-MeTHF (nM) | 0.36 | 0.27 | 0.14 | 0.22 | 0.29 | 0.26 | <0.001 |
| CSF Betaine (µM) | 1.76 | 1.70 | 1.83 | 2.04 | 2.05 | 2.37 | 0.005 |
| CSF Choline (µM) | 2.27 | 2.27 | 2.29 | 2.38 | 2.44 | 2.59 | 0.665 |
| RBC 5-MTHF (nM) | 803.6 | 641.9 | 611.9 | 887.2 | 1197.3 | 1063.3 | <0.001 |
| . | Control . | Day 8 . | Day 29 . | Day 36 . | Day 43 . | Day 50 . | p . |
|---|---|---|---|---|---|---|---|
| CSF 5-MTHF (nM) | 84.6 | 54.8 | 34.0 | 52.3 | 62.2 | 55.0 | <0.001 |
| CSF 5,10-MeTHF (nM) | 0.36 | 0.27 | 0.14 | 0.22 | 0.29 | 0.26 | <0.001 |
| CSF Betaine (µM) | 1.76 | 1.70 | 1.83 | 2.04 | 2.05 | 2.37 | 0.005 |
| CSF Choline (µM) | 2.27 | 2.27 | 2.29 | 2.38 | 2.44 | 2.59 | 0.665 |
| RBC 5-MTHF (nM) | 803.6 | 641.9 | 611.9 | 887.2 | 1197.3 | 1063.3 | <0.001 |
In the control group, the mean CSF 5-MTHF concentration was 84.6 nM. This was significantly increased compared to the concentrations seen in ALL patients at all timepoints, including baseline levels. The mean 5,10-MeTHF level for the control group was 0.36 nM. This was significantly different from ALL patients only on days 29 and 36 of treatment. There were no differences noted between CSF betaine and choline levels between ALL patients and controls.
There were four patients with ALL who experienced neurotoxicity (grade 3 of higher) related to methotrexate. There were no differences in CSF 5-MTHF, 5,10-MeTHF, betaine or choline levels between patients that experienced neurotoxicity compared to those who did not.
Conclusion: Children with ALL have a significant decrease in CSF 5-MTHF and 5,10-MeTHF levels following the initial administration of a single intrathecal dose of methotrexate. Despite additional intrathecal doses of methotrexate, these levels return to baseline by treatment day 36. This is accompanied by an increase in RBC 5-MTHF and CSF betaine. These data suggest that continued exposure of the CSF to methotrexate may induce systemic mechanisms of folate repletion.
1 van Haandel L, et al. Rapid Commun Mass Spectrom 2012; 26(14):1617-30.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal