Abstract
Background: Engraftment syndrome (ES) is an early complication after hematopoietic stem cell transplantation (HSCT) caused by a massive release of pro-inflammatory cytokines, products of degranulation, and oxidative metabolism, in which activated macrophage play a crucial role. Liposome-incorporated dexamethasone, dexamethasone palmitate (DP), is known to suppress activated macrophages by readily taken up via phagocytosis and strongly retained in the cytoplasm. In this retrospective study, we aimed to identify the incidence of ES, assess the efficacy and impact of DP treatment for ES on clinical courses after HSCT.
Patients & Methods: This retrospective study included 95 consecutive allogeneic HSCT for children (the median age at HSCT, 9.1 years; range, 0.5 - 18.0 years) with hematological malignancy performed in our institute between April 2006 and December 2014. The underlying diseases consisted of acute lymphoblastic leukemia (n=52), acute myelogenous leukemia (n=30), malignant lymphoma (n=8), and myelodysplastic syndrome (n=5). Sixty patients underwent bone marrow transplantation from human leukocyte antigen (HLA) identical family members (n=26), HLA haplo-identical family members (n=4), or unrelated volunteer donors (n=30), the other 35 patients received unrelated cord blood transplantation. Seventy-three patients were prepared by myeloablative conditioning regimen consisting of total body irradiation based regimen (n=57) and busulfan based regimen (n=16), the other 22 patients were prepared by reduced intensity conditioning. Forty-two patients received HSCT at advanced clinical stage; second remission after prior HSCT, third remission or non-remission. Current cohort contained 22 patients received second or more HSCT. The diagnosis of ES consists of Spitzer's criteria and specific bone marrow aspiration findings; proliferation of activated macrophage or phagocytosis. DP treatment (2.5 mg/m2/day I.V. for 3 consecutive days) indicated for all patients diagnosed as ES, and on demand DP treatment for refractory cases. Univariate analysis of overall survival (OS) was performed using the log-rank test, and Gray's test was used for group comparisons of cumulative incidences. Predictive factors with a p value <0.10 in the univariate analyses were set in the multivariate analysis (Cox hazard proportional model with the time-dependent covariate).
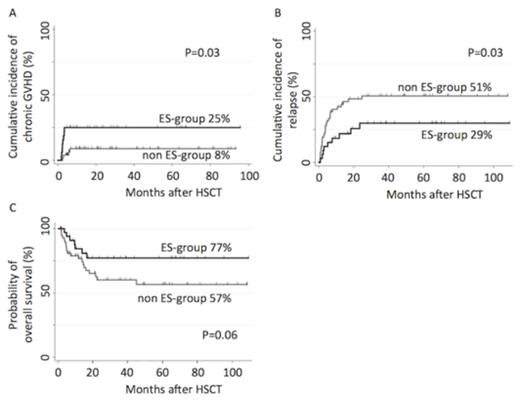
Results: Of 95 consecutive patients, 35 patients (38%) developed ES and were treated by DP; defied as ES-group. ES was resolved in 30 of 35 cases by DP treatment, however, 5 patients needed additional methylprednisolone treatment. The cumulative incidence of Cytomegalovirus (CMV) antigenemia in ES-group was significantly higher than that in non ES-group, whereas there was no difference in CMV infection, reactivation of Epstein-Barr virus, and virus associated hemorrhagic cystitis, between two groups. The cumulative incidence of neutrophil recovery at 42 days after HSCT were 94% (95% confidence interval [CI], 78 - 99%) for ES-group and 98% (95% CI, 88 - 100%) for non ES-group; the median time to engraftment was 19 days for ES-group and 17 days for non ES-group, respectively. The median follow-up of survivors was 48.9 months. The cumulative incidence of grade II-IV acute graft-versus-host disease (GVHD) for ES-group was comparable with that for non ES-group (12% versus 12% at 100 days, P=0.97). However, the ES-group was significantly associated with higher incidence of chronic GVHD (25% versus 8% at 4 years, P=0.03, Figure A), lower relapse rate (29% versus 51% at 4 years, P=0.03, Figure B), and higher OS (77% versus 57% at 4 years, P=0.06, Figure C). Moreover, multivariate analyses identified that developing ES as the independent favorable predictor for both relapse (hazard ratio [HR], 0.42; 95% CI, 0.20 - 0.90; P=0.03) and mortality (HR, 0.40; 95% CI, 0.17 - 0.95; P=0.04); simultaneously, advanced clinical stage (HR, 2.74; 95% CI, 1.41 - 5.32; P=0.003) was an independent poor predictor for relapse, and advanced clinical stage (HR, 6.51; 95% CI, 2.59 - 16.4; P<0.001) and age at HSCT >10 years (HR, 2.94; 95% CI, 1.31 - 6.42; P=0.009) were independent poor predictors for OS.
Conclusion: Our findings suggested that DP could control early post-transplant immune reaction such as ES without suppressing graft-versus-leukemia effect, which improves the clinical outcome for children with hematological malignancy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal