Abstract
Thymic-independent peripheral expansion of CD8+ cells derived from the graft in the initial stage of post-HSCT immune recovery is a well-known physiological event. Nevertheless, the description of symptomatic LGL leukemias and aggressive malignant cases in this setting may generate uncertainty, mostly in those cases in which the cytotoxic T lymphocyte expansion CTLe persists beyond the early transplantation period. We aimed to assess the nature of CTLe in adults during the post-alloHSCT period in a series of 154 patients with a long term surveillance. We studied the longitudinal kinetics of those expansions, their relation to clinical events, and their phenotypic and molecular features, including recently reported CTL leukemia-STAT3 mutations. In our study, trying to adhere to the WHO annotation of T-LGL, we considered two definitions for a CTL expansion: an absolute increase (≥ 2000 x109/L), and a relative expansion (a CD8/CD4 ratio ≥ 1.5), persisting more than six months in both cases. Persistent relative CTLe cases are frequent (49%) and related with timoglobulin prophylaxis (p≤0.001), acute graft versus host disease (GVHD, p=0.02), reduced intensity conditioning (p=0.04) and fungal and viral infections in the early post-HSCT. No differences in the number of serious infectious events from day 180 was found. Absolute CTLe are scarce (9%), related with chronic GVHD and absence of relapses. TCR rearrangement was reported as clonal and oligoclonal in the majority of patients with CTLe. We studied in a cross sectional manner with an extended immunophenotypic panel 17 patients: 5 patients with an absolute CTLe and 12 cases with a relative CTLe. A similar cytotoxic T αβ-effector phenotype was observed in all cases, with slight differences in the expression of CD25, CD16 and 1a. One patient with a relative CTLe expressed CD56 intensely: his ratio normalized at day 730 and no immune-related events were recorded. DNA stored during the post-alloHSCT setting was available from 68/75 relative CTLe patients (14/14 absolute CTLe cases). All of them went through molecular TCR rearrangement and STAT3 exon 21 mutations determination. In the relative CTLe cohort, TCR rearrangement was described as clonal, oligoclonal or polyclonal in 77%, 16% and 7%, respectively. Regarding absolute CTLe patients, TCR rearrangement was described as clonal in all the patients (n=14) of this subset. To increase the sensibility of the Sanger PCR, it was performed on DNA from CD3+ sorted cells in 54 out of 68 cases. No STAT3 mutation could be found in the CD3+ sorted fraction of relative or absolute defined CTLe. Not using an absolute threshold would establish a diagnosis of a persistent CTL expansion in 49% of our cohort of allo-transplanted patients. Additional diagnostic tools, as an effector phenotype, the presence of a NK marker or a monoclonal TCR rearrangement would not reduce significantly that percentage: CD57 was invariably expressed in CTLe cases, and 80% of our patients with expansions showed a TCR monoclonal pattern. STAT3 mutations resulting in persistent proliferation of CTL clones are a frequent event in large granular lymphocytic leukemia, and those clones have also been described in autoimmunity-driven disorders as acquired aplastic anemia and hypocellular myelodysplastic syndromes. We establish in this study the absence of exon 21 STAT3 mutations in the persistent CTL expansions found in a large series of patients with a long-term post-alloHSCT surveillance. The absence of STAT3 mutations and the CD8/CD4 declining longitudinal kinetics in the late period, supports its benign nature, expressed clinically by the null detrimental impact of these expansions on post-transplant outcome and/or serious infectious events.
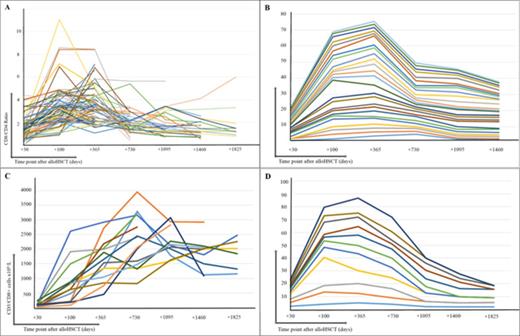
Relative and absolute CTLe kinetics. A) Linear representation over time of the CD8/CD4 ratio in the 75 patients with relative CTLe. B) Trend linear plot of 25 patients with a relative CTLe and a follow up of, at least, 1440 days from transplantation. Each line depicts a patientxs longitudinally measured CD8/CD4 ratio. Patients are grouped by similar pattern of ratio behaviour through follow up. The line "slope" depicts the magnitude of the change between time points. C) Linear representation over time of the CD3/CD8 count in PB in the 14 patients with an absolute CTLe. D) Trend linear plot illustrating the CD8/CD4 ratio behaviour of the 14 patients with an absolute CTLe.
Relative and absolute CTLe kinetics. A) Linear representation over time of the CD8/CD4 ratio in the 75 patients with relative CTLe. B) Trend linear plot of 25 patients with a relative CTLe and a follow up of, at least, 1440 days from transplantation. Each line depicts a patientxs longitudinally measured CD8/CD4 ratio. Patients are grouped by similar pattern of ratio behaviour through follow up. The line "slope" depicts the magnitude of the change between time points. C) Linear representation over time of the CD3/CD8 count in PB in the 14 patients with an absolute CTLe. D) Trend linear plot illustrating the CD8/CD4 ratio behaviour of the 14 patients with an absolute CTLe.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal