Abstract
An inherent difficulty in performing allogeneic transplantation in patients (pts) of African American (AA) descent is finding suitably matched 8/8 unrelated donors. In addition for complicated reasons studies have shown that AA have an inferior survival compared to suitable matched patients from different ethnic groups undergoing allogeneic transplantation. Post transplant cyclophosphamide (PTCy) administered on day + 3 and +4 given in combination with tacrolimus and mycophenolate mofetil (MMF) allows for the safe use of more mismatched transplants, including haploidentical transplants. This has created a new approach for patients who lack suitable donors and may allow more AA pts to proceed to transplant. At our institution, we have used PTCy as graft-versus-host-disease (GVHD) prophylaxis in patients undergoing reduced intensity conditioning (RIC) hematopoietic stem cell transplant (HSCT) from mismatched related and unrelated donors, and this group consisted largely of high-risk AA pts.
We retrospectively reviewed 16 pts who underwent HLA mismatched HSCT (5/10 to 8/10 HLA matches) at our institution between July 2012 and 2015. All patients received RIC HSCT with PTCy (days +3 and +4) along with tacrolimus and MMF for GVHD prophylaxis. Patients did not routinely receive growth factor support. Primary endpoints included time to neutrophil and platelet engraftment, length of hospital stay, and rates of acute and chronic GVHD. Secondary outcomes included time to relapse, transplant related mortality, and overall survival.
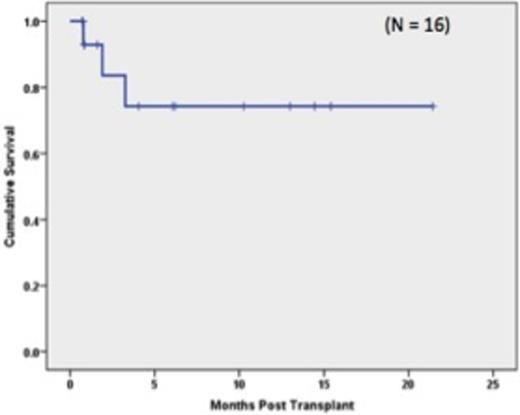
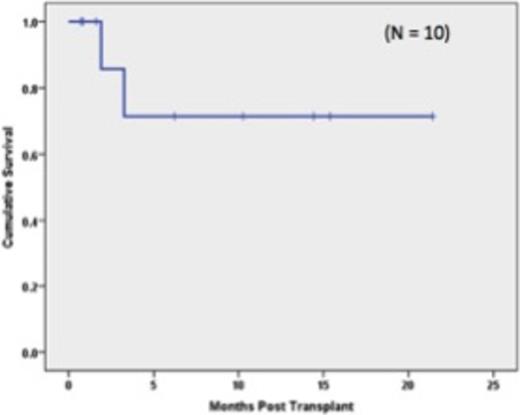
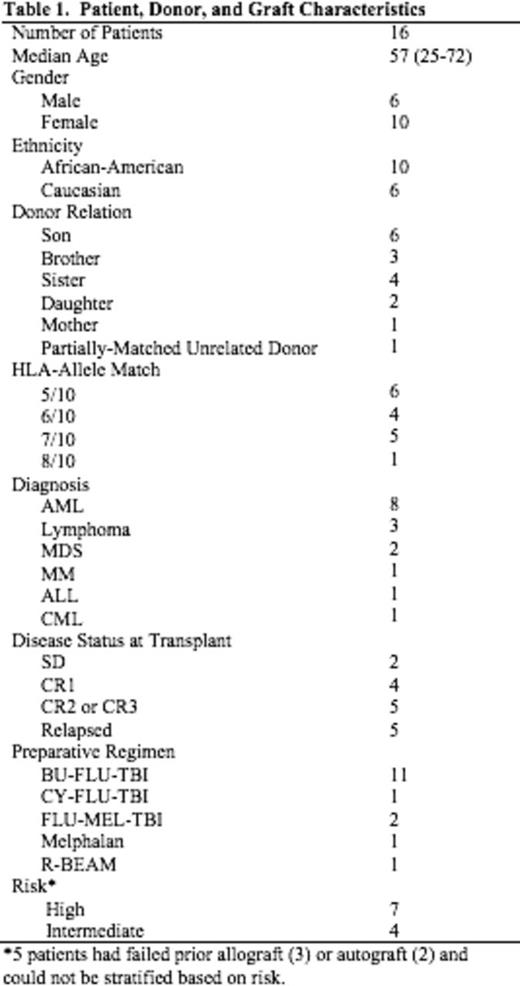
Table 1 shows the demographics of the 16 patients who were transplanted. The median age of the patients was 57 years (range: 25-72). Ten pts were of AA descent. Fifteen donors were haploidentical family donors; 6 were 5/10 matches, 4 were 6/10 matches, and 5 were 7/10 matches. One patient received a 8/10 unrelated donor transplant. Five patients had failed previous transplants which included 3 allogeneic and 2 autologous HSCT. Based on published risk stratification (Armand et al. Blood, 2012) of the remaining 11 pts, 7 pts were high risk and 4 were intermediate risk. Neutrophil engraftment (first of 3 days when ANC was ³500 cells/mm3) occurred a median of 19 days post transplant (range:12-22 days); Platelet engraftment (³20,000/µL without platelet transfusion) occurred a median of 21.5 days post-transplant (range: 14-37 days). Median length of hospitalization was 26 days (range: 22-81 days). Two pts failed to engraft after first HSCT. One of these pts had high levels of anti-HLA antibodies directed against her donor. Both pts went on to a second haploidentical transplant and engrafted their neutrophil counts at 13 days and 21 days; respectively. Grade II acute GVHD occurred in 6/16 patients. No patient developed grade III-IV aGVHD. Chronic GVHD was observed in 8/16 patients with severe chronic GVHD seen in one patient. With a median follow up of 160 days (range: 89-778 days) the Kaplan-Meier estimates of overall survival at one year were 81.3% for all transplanted pts (Figure 1) and 80% for the AA pts (Figure 2). Three pts died; 1 patient from an invasive fungal infection and 2 pts from relapse. One additional patient relapsed but continues on treatment.
Our experience suggests that the use of PTCy permits HLA mismatched transplantation, with overall survival of > 80% and acceptable rates of acute and chronic GVHD. Moreover, the severity of acute GVHD in patients who received PTCy post-transplant was limited, with no reported acute grade III-IV GVHD. In addition, the early outcomes of HLA mismatched transplantation in 10 African-American patients, a population that is often underrepresented in the donor registry, suggests that this approach may preferentially benefit AA pts from an expanded donor pool derived from utilization of partially HLA-matched donors.
Lum:Karyopharm Therapeutics Inc: Equity Ownership; Transtarget.Inc: Equity Ownership. Deol:Bristol meyer squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal