Abstract
Introduction: Hemostasis encompasses an ensemble of regulated and balanced interactions among platelets (and other blood cells), coagulation factors, the endothelium, and the hemodynamic forces of the circulation. Unfortunately, current bleeding assays assess only isolated aspects of this complex process. A single assay that incorporates all of the major components of hemostasis would be of significant value in the research and clinical settings. To that end, we leveraged our laboratory's expertise in microfluidic systems to construct an "in vitro" bleeding time. Specifically, we developed a perfusable "endothelialized" microvasculature model that, via a pressure-controlled microscale valve, sustains a controlled, localized vascular "injury" after which bleeding and hemostatic plug formation ensues. This system incorporates recalcified human whole blood with minimal anticoagulation with corn trypsin inhibitor (CTI), controlled perfusion at physiologic flow conditions, and a confluent monolayer of any endothelial cell type. When used in conjunction with fluorescently tagged antibodies or cellular dyes, the entire hemostatic process can be tracked in real time with single cell resolution. As human blood and cells can be used exclusively, this microfluidic system is ideal for studying healthy as well as patient samples. Here, we describe proof of concept experiments utilizing healthy human blood samples and a high-titer antibody that completely inhibits Factor VIII activity.
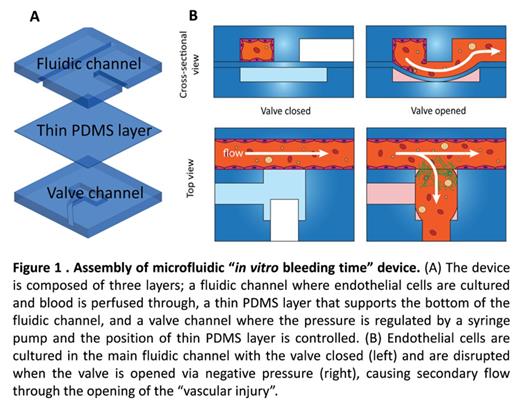
Materials and Methods: The polydimethysiloxane (PDMS)-based microfluidic bleeding time device is comprised of a fluidic, membrane valve, and channel layer (Figure 1A). The fluidic channel was pre-coated with collagen type 1 and fibronectin. Human umbilical cord vein or aortic endothelial cells were seeded in the main fluidic channel with membrane valve in the closed position (Figure 1B, valve closed), then cultured under flow to reach confluence. The "vascular injury" was introduced by providing negative pressure to pull down membrane valve at the middle of the device, where the endothelium is disrupted and an opening (i.e., the "bleed") is created (Figure 1B, valve opened). Human blood was collected in citrated buffer with CTI. Immediately after the injury, recalcified blood was perfused into the device at a shear rate of 500 s-1 and time-lapse images were taken by a confocal microscope. Wound width, bleeding time, and fluorescent intensity were measured and analyzed.
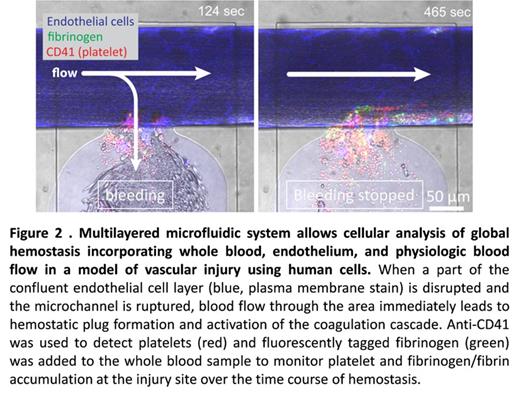
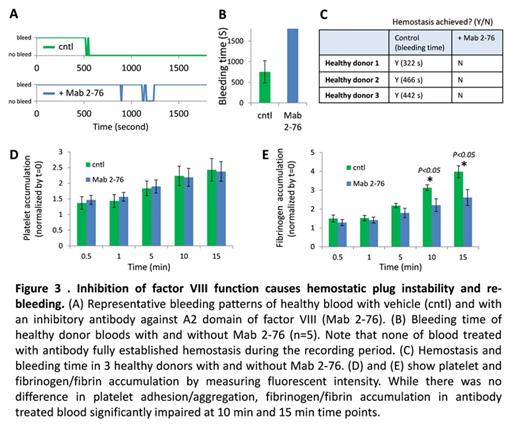
Results and Discussion: Here we demonstrate that our microfluidic system enables us to directly analyze microvascular injury, bleeding, and then hemostasis using human cells and blood. After the wound was introduced, blood flow in fluidic channel divided into main channel (Figure 2, horizontal flow) and into the collagen I-coated wound channel, i.e. "bleeding" (vertical flow). The wound width was varied depending on the valve size and pressure given, and ranged from 73.22 to 231.86 µm (n=55, average 132.67 ± 5.48 µm). The average time to cessation of blood flow into the wound channel (i.e. the "bleeding time") was 546.02 ± 38.26 seconds. Interestingly, individual wound width and bleeding time did not correlate (R2 = 0.087), suggesting that the time course of hemostasis was not affected by wound size. Immediately after the injury, platelet adhesion and aggregation led to the formation of hemostatic plug at the wound area followed by fibrin formation, which were visually confirmed via immunofluorescence (CD41) and fluorescent-tagged fibrinogen (Figure 2). In addition, fluorescently-tagged CD15, CD45, and Annexin V binding enabled us to visualize other cellular and molecular components pertinent in hemostasis. Finally, treating healthy blood with an antibody against A2 domain of Factor VIII (anti-A2 MAb 2-76) effectively induced a severe Hemophilia A phenotype. While platelet plug formation was unchanged, the antibody caused instability of clot and repeated re-bleeding. In fact, none of the MAb 2-76-treated conditions exhibited hemsostasis (n=5, the longest recording period 4865 sec), compared to the vehicle control. Also reduced fibrinogen/fibrin accumulation was observed in MAb 2-76-treated blood (Figure 3). Our ongoing studies will provide spatiotemporal insights into how different cellular and molecular components interact during hemostatic process and with use of patient samples, how they go awry in diseased conditions.
Jobe:Bayer: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees; CSL-Behring: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal