Abstract
Delivery of iron (Fe) to most cells occurs following the binding of diferric transferrin (Tf) to its cognate receptors on the cell membrane following which the Tf-receptor complexes are internalized via endocytosis. Iron is then released from Tf within endosomes by a combination of Fe3+ reduction by Steap3 and a decrease in pH (~ pH 5.5). Subsequently, Fe2+ is transported through the endosomal membrane by DMT1. In erythroid cells, more than 90% of Fe has to enter mitochondria where ferrochelatase, the final enzyme in the heme biosynthetic pathway that inserts Fe2+ into protoporphyrin IX, resides.
The intracellular path of iron from endosomes to ferrochelatase is still obscure or, at best, controversial. The prevailing opinion is that Fe, after its export from endosomes, spreads into the cytosol, from where the metal mysteriously finds its way into mitochondria. An opposing view is that the highly efficient transport of Fe toward ferrochelatase in erythroid cells requires a direct interaction between transferrin-endosomes and mitochondria ("kiss-and-run" hypothesis;Ponka Blood 89:1, 1997). Despite the longevity of the prevailing opinion, experimental evidence (Richardson et al. Blood 87:3477, 1996; Zhang et al. Blood 105:368, 2005; Sheftel et al. Blood 110: 125, 2007) only supports the latter hypothesis, which sees favorable reception among Cell Biologists (McBride BMC Biology 13:8, 2015).
Our laboratory has demonstrated, using both 2D and 3D live confocal imaging, that the intracellular Fe pathway in erythroid cells indeed involves a transient interaction of endosomes with mitochondria. To furtherdemonstrate the contact between these organelles, we have developed a novel method based on flow cytometry analysis ("flow sub-cytometry") of lysates obtained from reticulocytes with fluorescently labeled mitochondria (MitoTracker Deep Red; MTDR) and endosomes (Alexa Green Transferrin; AGTf). Using this strategy, we have identified three distinct populations: endosomes, mitochondria, and a population double-labeled with both fluorescent markers representing endosomes interacting with mitochondria. The size of the double-labeled population increases with the incubation time and plateaus in approximately 20 min.
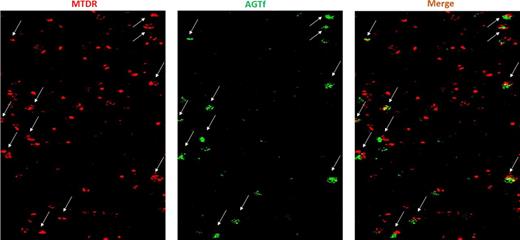
In this study, we examined whether reticulocyte mitochondria interact with Tf in a cell-free system. Lysates obtained by freeze-thawing of reticulocytes previously labeled with MTDR were incubated with AGTf for various time intervals. Examination of lysates by 2D confocal microscopy has revealed a time-dependent increase in the number of mitochondria being in contact with Tf-endosomes (fig 1: Images of mitochondria and endosomes; 20 min incubation with AGTf). This can be prevented by Fe2-Tf, but not by albumin, added to lysates. Moreover, the addition of unlabeled Fe2-Tf to reticulocyte lysates removed AGTf from mitochondria. We conclude that mitochondria from freeze-thawed reticulocyte lysates are associated with TfR that can reversibly bind Tf.
We have also embarked on uncovering molecular partners involved in the endosome-mitochondria interactions. Using co-immunoprecipitation and pull-down strategies, we have attempted to detect proteins interacting with the intracellular loops of DMT1 that could be candidates for interactions with mitochondria. The co-immunoprecipitated proteins were separated based on their molecular weights, stained using Coomassie and/or Silver gel and identified by mass spectrometry followed by western blotting. We co-immunoprecipitated (from murine eryhroleukemia [MEL] cells and reticulocytes lysates) proteins that were pulled down with DMT1. One of the proteins that we have recognized is the voltage-dependent anion channel (VDAC), which is located at the outer membrane of the mitochondrion (Graham, et al. Curr Top Dev Biol. 59: 87, 2004). The identity of DMT1 was confirmed by western blotting using specific antibodies against VDAC. These results further support the concept of the physical interaction between endosomes and mitochondria. To examine a possible role of DMT1-VDAC interactions in iron trafficking, we silenced the expression of VDAC in MEL cells followed by the measurement of 59Fe incorporation from 59Fe-Tf into heme. Our finding of decreased 59Fe incorporation into heme of MEL cells with silenced VDAC supports the idea that this outer-membrane mitochondrial protein is involved in the interaction with endosomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal