Abstract
Background: Approximately 60 - 80% of AML patients achieve a complete remission [CR] with one or two cycles of induction chemotherapy, leaving many patients with refractory AML [PIF]. Unfortunately, the majority of patients in CR1 ultimately relapse. With salvage therapy, only 30-50% achieve CR2. Those with PIF or relapsed AML have shortened survival and few therapeutic options.
Risk stratification is primarily based on karyotype, however other factors including age, initial white blood cell count, secondary AML and mutational status are also utilized to determine prognosis. HCT is an effective option for treatment of AML with intermediate/high risk features in CR1. It has also been utilized in refractory or relapsed disease. Advances in HCT over the last decade have improved overall survival (OS) and extended this option to older patients.
Our aim is to characterize outcomes after HCT for AML patients who are not in CR1.
Methods: We analyzed 136 AML patients who were not in CR1 at the time of HCT from 2004 - 2013. The conditioning regimen was fludarabine and myeloablative doses of PK targeted busulfan. IWG AML response criteria were used to define disease status at the time of transplant. Cytogenetic risk was based on the NCCN AML guidelines. OS is defined as the time from HCT until the time of death from any cause. Disease free survival (DFS) is defined as the time from HCT to the time of relapse or death from any cause.
Results: Disease status consisted of 74 (54.4%) in CR2, 6 (4.4%) in CR3 or beyond, 27 (18.9%) were PIF, 21 (15.4%) with relapsed AML (REL) that was treated but still present at time of transplant, and 8 (5.7%) who received either no treatment or a hypomethylating agent (HMA). Median age was 52.0 (21.8 - 72.5) years, and 80 (59%) were male. Time from most recent treatment to HCT was < 1 month in 8 (5.8%), 1-3 months in 75 (55.8%), >3 months in 50 (36.8%) and not applicable in 3. Ninety-six (70.6%) had de novo AML, while 40 (29.4%) had secondary AML. Cytogenetic risk was favorable in 32 (23.5%), intermediate in 57 (42%), poor in 40 (29.4%) and unknown in 7 (5.1%). Graft-versus-host disease prophylaxis was tacrolimus with methotrexate or sirolimus, or mycophenolate mofetil. Donors included 41 (30.2%) matched related, 2 (1.4%) mismatched related, 65 (47.8%) matched unrelated and 28 (20.6%) mismatched unrelated donors. Peripheral blood stem cells were used in 97.2% of cases.
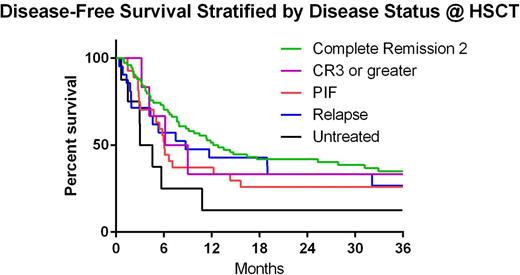
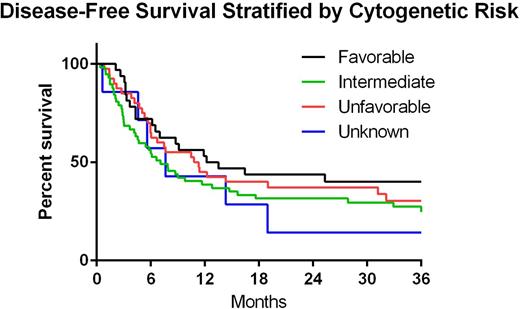
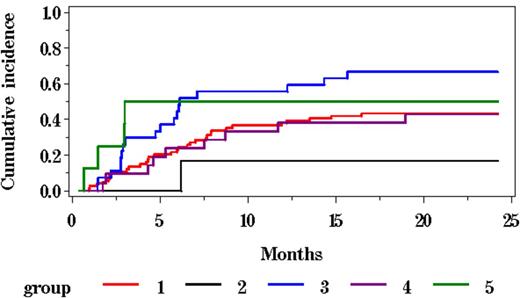
Two year OS, DFS, cumulative incidence (CI) of relapse and CI-NRM for all patients was 45.3%, 35.2%, 47.1% and 18.2%, respectively. Two-year DFS stratified by disease status at time of HCT was 41.9%, 33.3%, 25.9%, 33.3% and 12.5% in CR2, CR3 or beyond, PIF, REL and HMA, respectively(p=0.011 for CR2 vs HMA) (Figure 1). Two-year DFS stratified by cytogenetic risk was 43.8%, 31.6%, 37.1% and 14.3% in favorable, intermediate, poor and unknown, respectively (p>0.05) (Figure2). CI-Rel stratified by disease status was 43.2%, 16.7%, 66.7%, 42.9% and 50% in CR2, CR3 or greater, PIF, REL and HMA, respectively (Figure 3).
Conclusions: We analyzed 136 AML patients after undergoing HST outside of CR1 and the cumulative incidence of relapse at two years was 47%. Relapse was highest in those with primary induction failure or residual disease after either no or low intensity therapy. These data suggest that patients with active disease at the time of transplant fare worse than those who are transplanted in remission, highlighting the importance of effective upfront therapies in order to obtain the maximum potential benefit from HCT. Cytogenetic risk stratification did not significantly impact outcomes, although those with favorable risk cytogenetics trend towards higher 2-year DFS vs those with intermediate or poor-risk disease. Trials looking at the impact of maintenance therapy post-transplant may be valuable in this patient population.
| . | Disease Status @ HSCT . | ||||
|---|---|---|---|---|---|
| CR2 | CR3 or beyond | PIF | RES | HMA/untreated | |
| 2 years | 41.9% (30.6 - 52.8) | 33.3% (4.6 - 67.6) | 25.9% (11.5 - 43.1) | 33.3% (14.9 - 53.1) | 12.5% (0.7 - 42.3) |
| . | Disease Status @ HSCT . | ||||
|---|---|---|---|---|---|
| CR2 | CR3 or beyond | PIF | RES | HMA/untreated | |
| 2 years | 41.9% (30.6 - 52.8) | 33.3% (4.6 - 67.6) | 25.9% (11.5 - 43.1) | 33.3% (14.9 - 53.1) | 12.5% (0.7 - 42.3) |
| . | Cytogenetic Risk Group . | |||
|---|---|---|---|---|
| Favorable | Intermediate | Unfavorable | Unknown | |
| 2 years | 43.8% (26.5 - 59.8) | 31.6% (20.1 - 43.7) | 37.1% (22.5 - 51.8) | 14.3% (0.7 - 46.5) |
| . | Cytogenetic Risk Group . | |||
|---|---|---|---|---|
| Favorable | Intermediate | Unfavorable | Unknown | |
| 2 years | 43.8% (26.5 - 59.8) | 31.6% (20.1 - 43.7) | 37.1% (22.5 - 51.8) | 14.3% (0.7 - 46.5) |
| . | Cumulative Incidence of Relapse . | ||||
|---|---|---|---|---|---|
| CR2 (1) | CR3 or beyond (2) | PIF (3) | REL (4) | HMA/untreated (5) | |
| 2 years | 43.2% (32.2 - 54.6) | 16.7% (0.0 - 53.5) | 66.7% (48.1 - 82.9) | 42.9% (23.0 - 64.0) | 50.0% (18.1 - 81.9) |
| . | Cumulative Incidence of Relapse . | ||||
|---|---|---|---|---|---|
| CR2 (1) | CR3 or beyond (2) | PIF (3) | REL (4) | HMA/untreated (5) | |
| 2 years | 43.2% (32.2 - 54.6) | 16.7% (0.0 - 53.5) | 66.7% (48.1 - 82.9) | 42.9% (23.0 - 64.0) | 50.0% (18.1 - 81.9) |
Sweet:Novartis Pharmaceuticals: Speakers Bureau; Ariad Pharmaceuticals: Consultancy, Speakers Bureau; Karyopharm Therapeutics Inc: Research Funding; Incyte: Research Funding. Lancet:Celgene: Consultancy, Research Funding; Seattle Genetics: Consultancy; Boehringer-Ingelheim: Consultancy; Pfizer: Research Funding; Kalo-Bios: Consultancy; Amgen: Consultancy. Perkins:PDL Biopharma: Research Funding. Field:PDL Biopharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal