Abstract
Genome editing has the potential to provide long-term therapeutic gene expression in vivo. We have previously demonstrated efficient editing in a mouse model of hemophilia B through liver-directed adeno-associated viral vector (AAV) delivery of a zinc finger nuclease (ZFN) pair and a corrective donor. We determined that homology is not necessary to achieve efficient levels of genome editing in adult mice, consistent with the fact that quiescent cells, including adult hepatocytes, are not thought to be amenable to homology directed repair (HDR). As a consequence of the donor containing a splice acceptor, both HDR and homology independent vector integration are capable of driving human factor 9 (hF.IX) expression. In this study we sought to determine whether hF.IX expression in mice treated as neonates, undergoing substantial hepatocyte proliferation, is predominantly the result of HDR or homology independent genome editing. Provided the efficacy is not substantially reduced, an HDR dependent approach would impose additional constraints on targeting.
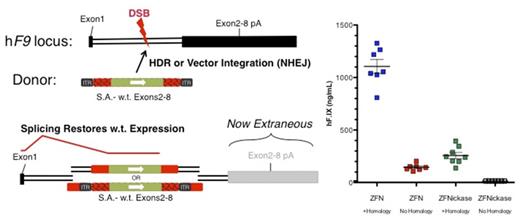
Treatment of neonatal hF9mut mice (harboring the ZFN target site) with 1x1011 vg AAV8-ZFN and 5x1011 vg AAV8-Donor via retro-orbital injection resulted in a drastic difference in hF.IX expression between donors with and without homology 10 weeks post injection (Homology: 1531 ± 174.5 ng/mL vs. No-homology: 146.1 ± 5.8 ng/mL; n=12 and 7, respectively). We next asked whether HDR could be stimulated even more specifically through the induction of DNA single strand breaks at the target site. We treated neonatal mice with homologous or non-homologous donors, as well as ZFNs or ZFNickases (in which one FokI nuclease domain was inactivated with the D450A mutation). ZFNickases were indeed active, resulting in ~250 ng/mL hF.IX 4 weeks post injection (Figure 1). Interestingly, we could not detect hF.IX in mice treated with ZFNickase and no-homology donor (LOD: 15ng/mL). To rule out the possibility that this was simply due to the lower efficacy of ZFNickases compared to ZFNs, we increased the ZFNickase dose 4 fold. Four weeks post treatment, we observed substantial levels of hF.IX in mice treated with homologous donor (2041 ± 269 ng/mL) and were again unable to detect hF.IX in mice treated with the non-homologous donor (n=10 and 7, respectively). These data point to homology directed repair as the primary mechanism of protein production for genome editing in neonatal mouse liver, and suggest improvements in both efficacy and specificity can be made through deeper understanding of the molecular requirements of this approach.
Anguela:Spark Therapeutics, Inc.: Employment, Equity Ownership, Patents & Royalties. Doyon:Sangamo BioSciences: Employment. Wechsler:Sangamo BioSciences: Employment. Paschon:Sangamo BioSciences: Employment. Davidson:Spark Therapeutics: Consultancy. Gregory:Sangamo BioSciences: Employment. Holmes:Sangamo BioSciences: Employment. High:Spark Therapeutics: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal