Abstract
Background: We previously reported potent anti-tumor activity of the oral BRAF inhibitor vemurafenib in patients with relapsed or refractory BRAF mutant hairy cell leukemia (HCL) (Park et al. ASH 2014). According to the study design, patients whose disease relapsed following the initial treatment were allowed to be re-treated with vemurafenib. Here we report the clinical outcome of patients who were retreated with vemurafenib at relapse following initial treatment, as well as the result of genomic analysis that provided an insight into mechanisms of resistance to BRAF inhibition in HCL.
Patients and Methods: Patients with BRAF mutant HCL who were refractory or resistant to purine analogs, or who had ≥2 relapses with an indication for treatment (ANC ≤1.0, HGB ≤10, or PLT ≤100K) were enrolled. Eligible patients received vemurafenib 960mg twice daily for 3 months. Bone marrow (BM) evaluations were performed after 3 months to assess response. Patients with partial (PR) or complete response (CR) with detectable minimal residual disease were allowed to receive vemurafenib for up to 3 additional months. After a maximum of 6 months of therapy, patients were observed with monthly CBC. At disease relapse with peripheral blood (PB) counts low enough to meet the initial eligibility criteria, re-treatment with vemurafenib was allowed until disease progression or unacceptable toxicity. Serial PB and/or BM samples were collected for targeted next-generation sequencing analysis of a 300-gene panel to detect contributors to resistance and genes collaborating with BRAF mutations in HCL.
Results: 26 patients have been enrolled. 2 patients discontinued treatment before response assessment: 1 patient due to primary refractory disease to vemurafenib and 1 patient due to grade 3 photosensitivity. 24 patients completed at least 3 months of treatment, and therefore are available for efficacy evaluations. Of the 24 evaluable patients, all patients achieved response (10 CR and 14 PR) with the overall response rate of 100% when assessed after 3 months of vemurafenib.
With the median follow up of 11.7 months (range, 1.3 - 25.4 months), 7 patients experienced disease relapse (3 previous CR and 4 PR). Of the 7 relapse patients, 6 met re-treatment criteria and restarted vemurafenib. 4 of the 6 patients regained response (all PR) with complete hematologic recovery and remain on therapy. 2 patients discontinued re-treatment before response assessment: 1 patient due to grade 2 photosensitivity and fatigue, and 1 patient due to resistant disease with refractory cytopenia and a rapid increase in splenomegaly.
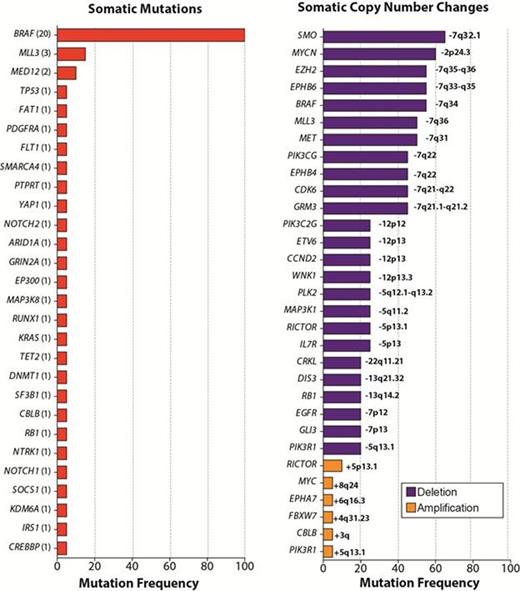
Targeted genomic analysis in 20 patients pre-vemurafenib revealed at least 1 somatic alteration coexisting with the BRAF V600E mutation in every patient, including deletion of 7q in more than half of patients and recurrent mutations in MLL3 and MED12 (Figure). Genomic analysis of the patient with de novo resistance to vemurafenib identified a missense mutation in IRS1 (Insulin Receptor Substrate 1; IRS1 P1201S) in addition to the BRAF V600E mutation. Functional characterization of the IRS1 P1201S mutation in vitro revealed potent induction of MAP kinase and PI3K-AKT signaling by the IRS1 mutant relative to wildtype, consistent with prior knowledge that IRS1 activates both MAP kinase and PI3K-AKT signaling. These data suggest that bypass activation of ERK and parallel activation of the AKT pathway contributed to de novo vemurafenib resistance.
In the patient with acquired resistance to vemurafenib, genetic analysis of pretreatment, remission and relapse PB mononuclear cells revealed emergence of 2 separate, activating subclonal KRAS mutations at relapse. The mutations in KRAS were not seen at pretreatment or at remission. Activating RAS mutations are well known mediators of vemurafenib resistance in BRAF V600E-mutant malignancies, and, in this case, the detection of KRAS mutations coincided with clinical relapse and insensitivity to vemurafenib.
Conclusions: Despite high response rates after a short course of vemurafenib in most patients, we observed de novo and acquired resistance to vemurafenib. Serial genomic analysis revealed ERK-dependent and independent mechanisms of BRAF inhibitor resistance in HCL. Our data provide the first insights into genetic mechanisms of RAF inhibitor resistance in HCL and suggest combinatorial therapeutic strategies that may have a role in the therapy of HCL.
Park:Amgen: Consultancy; Juno Therapeutics: Other: Advisory Board, Research Funding; Genentech: Research Funding. Off Label Use: Vemurafenib in HCL. Stone:Agios: Consultancy; AROG: Consultancy; Juno: Consultancy; Celgene: Consultancy; Abbvie: Consultancy; Celator: Consultancy; Merck: Consultancy; Karyopharm: Consultancy; Amgen: Consultancy; Pfizer: Consultancy; Roche/Genetech: Consultancy; Sunesis: Consultancy, Other: DSMB for clinical trial; Novartis: Research Funding. Rai:Nash Family Foundation: Research Funding; Karches Family Foundation: Research Funding; Nancy Marks Family Foundation: Research Funding; Leon Levy Foundation: Research Funding. Altman:Seattle Genetics: Other: Advisory board; Ariad: Other: Advisory board; Spectrum: Other: Advisory board; Novartis: Other: Advisory board; BMS: Other: Advisory board; Astellas: Other: Advisory board; assistance with abstract preparation. Levine:Foundation Medicine: Consultancy; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal