Abstract
Introduction: Acute myeloid leukemia (AML) comprises 5% of pediatric cancers and reports 5 year event free survival (EFS) of approximately 50% and overall survival (OS) of 60-70% following intensive multi-agent chemotherapy. Anthracyclines have been the cornerstone in upfront treatment of AML since the 1960's and continue to be used today in upfront trials for AML. Anthracycline induced cardiotoxicity remains a significant contributor to late morbidity/mortality in children and young adults with AML. The cardioprotectant dexrazoxane (Zinecard) can be used as prophylaxis to diminish the risk for cardiomyopathy but whether it affects the risk of relapse in pediatric AML is unproven. Our institution adopted the use of dexrazoxane prior to administration of any non-liposomal anthracycline for all patients in 2011. We are therefore reporting the differences in cardiac and treatment outcomes in children and young adults with AML treated with and without dexrazoxane from 2008 to 2013.
Methods: We performed a retrospective chart review of children ages 0 to 21 years who received their therapy for AML at the Children's Hospital of Wisconsin (CHW) between January 1, 2008 and December 31, 2013. This study was approved by the CHW institutional review board prior to our data collection. Descriptive statistics were used to describe the study population. Echocardiogram statistics were generated with each model based on the least squares means and then transformed back to its original unit. A log rank test was used to detect differences between populations. Based on number of patients, a hazard ratio of 3 will achieve 85% power to detect a significant difference between groups.
Results: Forty-four patients with AML were treated at CHW during 2008 - 2013 with 28 (64%) receiving dexrazoxane cardioprotectant and 16 (36%) did not. The median age at diagnosis was 8.1 years (5 months - 21.7 years) and 55% (n=24) were female. Six (14%) patients underwent transplantation in first complete remission and 6 (14%) underwent transplantation following relapse. No patients had history of cardiac disease, cardiac surgery or hypertension prior to treatment.
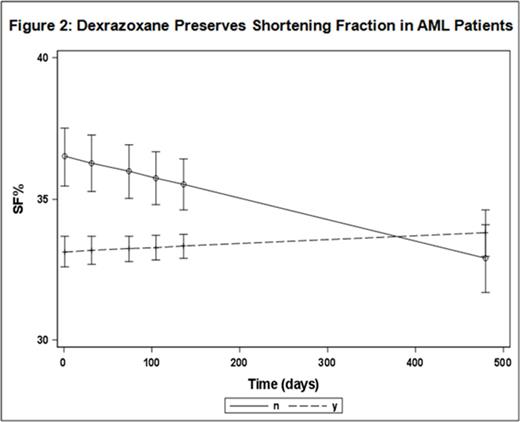
We identified no statistical difference in relapse free survival (RFS) p>0.40), EFS (p>0.48) or OS (p>0.53) between groups. However, there was a significant decrease in the ejection fraction (EF %) (p=0.0018) and a significant decrease in shortening fraction (SF %) (p=0.0108) trends over time in the non-dexrazoxane group compared to patients who received dexrazoxane (Figures 1 & 2).
Conclusion: Utilization of the cardioprotectant dexrazoxane prior to anthracycline chemotherapy in pediatric patients with AML demonstrated no significant difference in RFS or OS relative to our institutional controls and appeared to improve cardiac function. Further studies are needed to confirm these findings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal