Abstract
Calreticulin (CALR) mutations disrupting domains of the protein that regulate its intracellular trafficking were discovered in patients with myeloproliferative neoplasms (MPN) without JAK2 mutations and are associated with JAK2 activation (Klampt et al, NEJM 2013;369:2379; Nangalia et al, NEJM 2013;369:2391). Whether JAK2 mutations are associated with altered CALR functions is unknown.
We previously reported that erythroid cells (Erys) generated in-vitro from JAK2+-polycythemia vera (PV) patients do not respond to dexamethasone (Dex) because in these cells the glucocorticoid receptor (GRα) is constitutively retained in the nucleus (Varricchio et al, Blood 2011;118:425). This was explained by the high frequency found in PV patients of the A3669G GR polymorphism that increases expression of GRβ, the isoform responsible for nuclear retention of GRα. The A3669G frequency is also increased in primary myelofibrosis (PMF) and, when associated with JAK2 mutations, predicts poor survival (Poletto et al, Blood 2012;120:3112). Although the relationship between GR and CALR in Erys has not been defined as yet, studies in animal models indicate that in non-erythroid cells CALR regulates GRα nuclear export. This suggested that CALR may favor nuclear export of GRα, antagonizing GRβ, also, in Erys, and that this function may be impaired both by JAK2 and CALR mutations found in MPN. To test this hypothesis, we compared content, localization and association of CALR and GR in Erys expanded in-vitro from normal sources (NS) or from JAK2 + and CALR + MPNs.
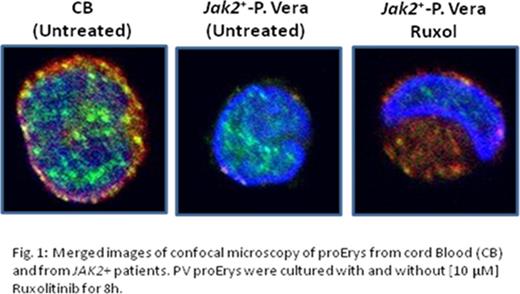
By Western Blot (WB), NS and MPN Erys contained comparable levels of CALR and GR. By FACS, in NS Erys cell-surface CALR expression increased from the proerythroblast (proEry) to the polychromatic (polyEry) stage [mean fluorescent intensity (MFI)=215±51 vs 460±125, p<0.05]. Conversely, confocal microscopy of permeabilized cells (to remove cell-surface proteins) detected greater levels of CALR in the cytoplasm of proErys (Fig 1) than in that of polyErys (not shown). CALR was never detected in the nuclei of NS Erys. Thus, maturation of normal Erys is associated with cytoplasm to cell surface trafficking of CALR. By contrast, JAK2+ -PV proErys expressed greater cell-surface levels of CALR than NS proErys (MFI=304±69, p<0.05) and barely detectable levels of CALR in the cytoplasm (Fig 1). Cytoplasmic levels of CALR were also barely detectable in JAK2 + and CALR + PMF proErys (not shown). These results indicate that CALR trafficking is altered in JAK2 + and CALR + proErys alike.
By WB and confocal microscopy in NS proErys GRα was prevalently localized in the nuclei or cytoplasm, depending on whether cells had been exposed to Dex (which provides nuclear export signals) or erythropoietin/stem cell factor (that by activating Ca2+ signaling presumably stimulates CALR). In the cytoplasm, GR was co-localized with CALR in proximity of the nuclear membrane. Multi-regression analyses of 410 Pro-Erys compared single CALR and total and cytoplasmic GR signals as independent parameters against the merged signals (co-localization) as dependent parameter. This showed that co-localization correlated significantly with cytoplasmic levels of both CALR and GR but that the greatest p values were observed for CALR, suggesting that CALR is the driving force in determining the interaction between the two proteins.
By contrast, in MPN proErys GRα was localized prevalently in nuclei and CALR remained barely detectable in the cytoplasm and their localization was unaffected by treatments with Dex or erythropoietin/stem cell factor. Treatments of JAK2+ Erys with ruxolitinib increased total CALR levels (by 30%, p<0.05) and reduced its cell-surface expression (MFI=304±69 vs 220±48, p<0.05), increasing that in the cytoplasm of proErys (Fig 1). Although this treatment did not alter expression of GRβ, which remained high, it restored the presence of GRα in the cytoplasm where it was associated with CALR in the perinuclear region (Fig. 1) making JAK2+ Erys responsive to Dex (MTT incorporation 0.334±0.081 vs 0.224±0.045 with and without Dex, p<0.05), suggesting that impairment of the nuclear export signal provided by CALR cooperates with GRβ in retaining GRα in the nucleus of proErys from MPNs.
These results provide the first indication that cellular distribution, and possibly GRα nuclear export functions, of CALR are altered in erythroid cells from MPN patients irrespective of their mutation status.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal