Abstract

T-cell prolymphocytic leukemia (T-PLL) is a rare hematologic disease characterized by a T-cell phenotype, rapid progression, and poor prognosis with median survival of less than a year. Alemtuzumab-based chemotherapy has increased the rate of complete remissions but these are often short-lived and allogeneic transplant is considered the only curative therapy. In recent studies, JAK3 activating mutations have been identified in T-cell cancers, with T-PLL having the highest rate of JAK3 mutations (30 - 42%). As such, T-PLL is a model disease for evaluating the utility of JAK3 inhibitors. We present a case of a 64 year old man with relapsed-refractory T-PLL. He was initially treated with alemtuzumab and obtained complete response and was consolidated with matched unrelated donor stem cell transplant. His disease stayed in remission for approximately 1.5 years before relapse, which was then treated with a clinical trial of romedepsin-lenalidomide (partial responses then progression at 6 months) and later alemtuzumab. Due to complications of myelosuppression and CMV reactivation, his treatment was interrupted leading to disease progression (Figure 1). The doubling time of lymphocyte count was approximately 20 days and over a span of 60 days the lymphocyte count rose from 8 x 109/L to 68 x 109/L. Exon sequencing showed a JAK3 mutation. The patient consented to and was treated with FDA-approved tofacitinib (initially 5 mg BID, increased to 10 mg BID after 15 days of treatment). An initial decrease in lymphocyte count was followed by progression. In vitro treatment of the patient's cells showed modest effects of tofacitinib and ruxolitinib as single agents, in the range of doxorubicin, but synergy between the agents (Figure 2). After 40 days of treatment with tofacitinib and with a lymphocyte count of 150 x 109/L, ruxolitinib (5mg BID) was added. Over the 60 days since dual inhibition was started, the lymphocyte count has stabilized. The patient has remained completely asymptomatic during treatment with tofacitinib and ruxolitinib. Neutrophil count has remained normal. Platelet count and hemoglobin have however declined from ~50 x109/L to ~30 x109/L and from 11 g/dL to 8.1 g/dL respectively, since the introduction of ruxolitinib. The stabilization in lymphocyte count confirms the clinical activity of JAK inhibitors in T-PLL as suggested by the presence of JAK3 mutations and by in-vitro assays. It also suggests clinical synergy between ruxolitinib and tofacitinib in this setting. Prospective studies of JAK inhibitors in PLL patients with formal dose-finding studies are needed.
The overall trend of lymphocytes following disease refractory to or complicated by multiple lines of treatment. The patient demonstrated rapidly rising LDH and lymphocyte count. Following administration of tofacitinib (red arrow) and ruxolitinib (blue arrow), lymphocyte counts stabilized.
The overall trend of lymphocytes following disease refractory to or complicated by multiple lines of treatment. The patient demonstrated rapidly rising LDH and lymphocyte count. Following administration of tofacitinib (red arrow) and ruxolitinib (blue arrow), lymphocyte counts stabilized.
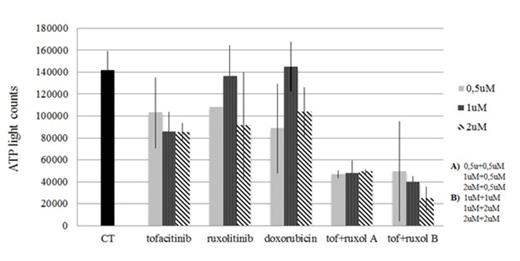
Level of ATP in patient cells treated for 24 hours with control, tofacitinib (tof), ruxolitinib (ruxol), doxorubicin or a combination of tofacitinib and ruxolitinib. There is synergistic depression of cell metabolism and growth for cells treated with both tofacitinib and ruxolitinib.
Level of ATP in patient cells treated for 24 hours with control, tofacitinib (tof), ruxolitinib (ruxol), doxorubicin or a combination of tofacitinib and ruxolitinib. There is synergistic depression of cell metabolism and growth for cells treated with both tofacitinib and ruxolitinib.
Off Label Use: Jak inhibitors in TCL. van Besien:Miltenyi Biotec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal