Abstract
Acute myeloid leukemia (AML) is primarily treated with chemotherapy, but the 5-year survival rate has only marginally increased over the past few decades, highlighting the need for novel targeted therapy. We have reported elevated expression of BCL-2 in AML and that BCL-2 inhibition by ABT-199 (ABT, venetoclax) induced on-target apoptosis, which could be predicted by BH3 profiling (Pan, et al., Cancer Discovery, 2014). ABT also showed encouraging clinical activity in relapsed/refractory AML (Konopleva et al., ASH 2014), yet MCL-1-mediated resistance may limit its use as monotherapy.
p53 mutations are relatively rare in AML, but its functions are often suppressed by overexpressed MDM2 protein. Since p53 and BCL-2 family proteins are central regulators of apoptosis, we asked whether concurrent BCL-2 inhibition and p53 activation (by MDM2 inhibitor) could overcome resistance to apoptosis and synergistically induce apoptosis in AML cells.
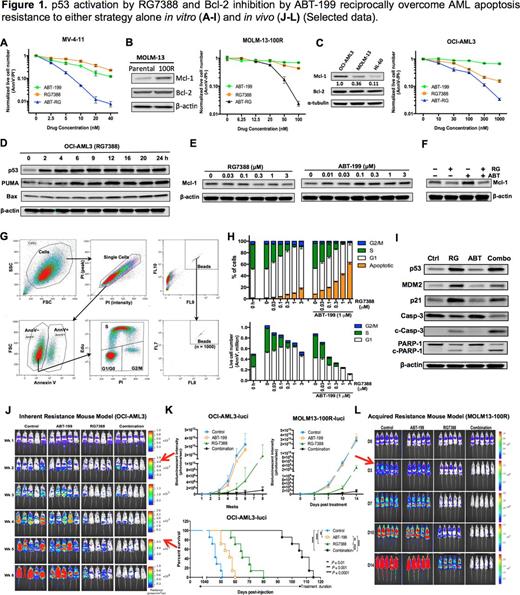
The novel MDM2 inhibitor RG7388 (RG, Idasanutlin) robustly activated p53 and induced growth inhibition and apoptosis of AML cells in a p53-dependent manner. p53 activation by RG also synergized with BCL-2 inhibition in killing ABT-sensitive cell lines such as MOLM-13 or MV-4-11 (Fig. 1A). After long-term exposure to escalating doses of ABT, initially sensitive cells upregulated MCL-1 and acquired resistance. Nonetheless, the acquired resistance could be effectively abrogated by RG (Fig. 1B). OCI-AML3 cells express a high basal level of MCL-1, and are inherently resistant to ABT. Concurrent p53 activation and BCL-2 inhibition induced synergistic apoptosis and overcame the inherent ABT resistance (Fig. 1C).
Next, we studied the underlying mechanisms. p53 activation by RG increased the expression of PUMA and BAX (but not NOXA, Fig. 1D), which are able to counteract MCL-1. In addition, p53 activation quickly dephosphorylated ERK2 and downregulated MCL-1 (Fig. 1E). Surprisingly, ABT increased ERK2 phosphorylation and upregulated MCL-1 (Fig. 1E). Because active ERK2 phosphorylates and stabilizes MCL-1, these results indicate that the observed changes in MCL-1 levels could be attributed to ERK2 phosphorylation status. Consistently, ERK2 dephosphorylation by MEK inhibitors quickly reduced MCL-1. Most importantly, ABT-induced ERK2 phosphorylation and MCL-1 upregulation could be reversed by p53 activation (Fig. 1F). These mechanistic studies provide insights into how p53 activation overcomes acquired/inherent resistance to BCL-2 inhibition.
OCI-AML3 cells are relatively resistant to p53 activation by RG. We used concomitant Annexin V staining, EdU pulsing and PI staining to simultaneously analyze apoptosis induction and cell cycle distribution of live cells (Fig. 1G). p53 activation by RG induced cell accumulation in G1 phase, while little apoptosis occurred (Fig. 1H). Addition of ABT dramatically increased apoptosis, reduced G1-arrested cells, and boosted apoptotic hallmarks like the cleavage of caspase-9, -3 and PARP-1 (Fig. 1H-I). ABT did not affect p21 expression and cell cycle distribution, and p53 activation induced robust expression of p21 and G1 arrest. Furthermore, p21 knockdown significantly decreased G1-arrested cells and increased apoptosis following p53 activation, indicating that p21 upregulation and G1 arrest mediate apoptosis resistance to p53 activation. Nonetheless, addition of ABT effectively shifted cell response from G1 arrest to apoptosis, suggesting BCL-2 inhibition can reciprocally overcome apoptosis resistance to p53 activation.
Next, we tested the combination in two AML mouse models. In an OCI-AML3-derived mouse model (with inherent resistance to ABT or RG), ABT or RG prolonged survival by 10 or 19 d, respectively, while the combination prolonged mouse survival by 61 d (Fig. 1J-K). Currently, we are also following the survival of mice in a MOLM-13 acquired resistance model. Early results indicate the tumor burden in combination group is <1/100 of that in control/ABT groups and ~1/20 of that in the RG group at day 14 (Fig. 1K-L).
In summary, BCL-2 inhibition by ABT and p53 activation by RG can reciprocally overcome resistance to apoptosis encountered by using either treatment alone in vitro and in vivo. Since both BCL-2 and MDM2 overexpression are associated with poor prognosis in AML, the proposed combination of the two clinical-stage compounds could have considerable clinical impact in relapsed/refractory AML.
Leverson:AbbVie: Employment, Equity Ownership. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding. Nichols:Roche Pharma: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal