Abstract
Introduction: Single-agent carfilzomib has shown encouraging activity in patients with relapsed and refractory multiple myeloma who harbor high-risk cytogenetic abnormalities (Jakubowiak et al, Leukemia 2013;27:2351-56). The phase 3 study ASPIRE (NCT01080391; N=792 patients) demonstrated that progression-free survival (PFS) was significantly improved with carfilzomib, lenalidomide, and dexamethasone (KRd), compared with lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma (RMM) (Stewart et al, N Engl J Med 2015;372:142-52). We present a pre-planned subgroup analysis of the efficacy and safety of KRd vs Rd in the ASPIRE study according to baseline cytogenetic risk status.
Methods: Adults with RMM (1-3 prior lines of therapy) were eligible. Patients were randomized (1:1) to KRd or Rd. Treatment was administered in 28-day cycles. Patients in the KRd arm received carfilzomib as a 10-minute intravenous infusion on days 1, 2, 8, 9, 15, and 16 (starting dose, 20 mg/m2 on days 1 and 2 of cycle 1; target dose, 27 mg/m2 thereafter) during cycles 1-12; carfilzomib was omitted on days 8 and 9 during cycles 13-18 and was discontinued after 18 cycles. All patients received lenalidomide 25 mg on days 1-21 and dexamethasone 40 mg on days 1, 8, 15, and 22. The primary end point was PFS. Secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), health-related quality of life, and safety. Cytogenetic risk status was assessed using fluorescence in situ hybridization. The high-risk group consisted of patients with the genetic subtype t(4;14) or t(14;16) or with deletion 17p in ≥60% of plasma cells, according to central review of bone marrow samples obtained at study entry. The standard-risk group consisted of all other patients with known baseline cytogenetics. The cutoff value of 60% for the proportion of plasma cells with deletion 17p was used on the basis of recommendations from the International Myeloma Workshop Consensus Panel 2 (Munshi et al. Blood 2011;117:4696-700).
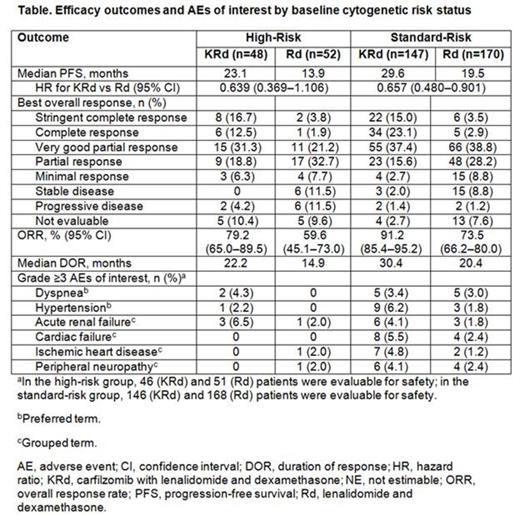
Results: A total of 792 patients were randomized to KRd (n=396) or Rd (n=396). In patients with known baseline cytogenetics, baseline cytogenetic risk status was similar between the treatment arms (high-risk: KRd, 24.6%; Rd, 23.4%; standard-risk: KRd, 75.4%; Rd, 76.6%). Efficacy outcomes by cytogenetic risk status are presented in the Table. Median PFS in the high-risk group (n=100) was 23.1 months (95% confidence interval [CI]: 12.5-24.2) for KRd vs 13.9 months (95% CI: 9.5-16.7) for Rd (hazard ratio [HR]: 0.639; 95% CI: 0.369-1.106). Median PFS in the standard-risk group (n=317) was 29.6 months (95% CI: 24.1-not estimable) for KRd vs 19.5 months (95% CI: 14.8-26.0) for Rd (HR: 0.657; 95% CI: 0.480-0.901). ORRs were 79.2% (KRd) vs 59.6% (Rd) in the high-risk group, and 91.2% (KRd) vs 73.5% (Rd) in the standard-risk group. In the high-risk group, 29.2% (KRd) and 5.8% (Rd) of patients achieved a complete response (CR) or better, including 16.7% (KRd) and 3.8% (Rd) with a stringent complete response (sCR). In the standard-risk group, 38.1% (KRd) and 6.5% (Rd) of patients achieved ≥CR, including 15.0% (KRd) and 3.5% (Rd) with an sCR. Median DOR in the high-risk group was 22.2 months for KRd vs 14.9 months for Rd. Median DOR in the standard-risk group was 30.4 months for KRd vs 20.4 months for Rd. The rate of grade ≥3 adverse events was 89.1% (KRd) vs 78.4% (Rd) in the high-risk group, and 85.6% (KRd) vs 84.5% (Rd) in the standard-risk group. Rates of grade ≥3 adverse events of interest (dyspnea, hypertension, acute renal failure, cardiac failure, ischemic heart disease, and peripheral neuropathy) by cytogenetic risk status are presented in the Table.
Conclusion: In patients with high-risk cytogenetics, treatment with KRd resulted in a median PFS of nearly 2 years, which was a 9-month improvement relative to that in patients treated with Rd. Treatment with KRd also led to a 10-month improvement in median PFS vs Rd in patients with standard-risk cytogenetics; similar reductions in the risk of progression or death with Kd vs Vd were observed in both cytogenetics risk groups. Treatment with KRd also led to higher response rates, greater response depth, and a longer DOR compared with Rd in patients with high- or standard-risk cytogenetics. KRd had a favorable benefit-risk profile in patients with RMM, irrespective of baseline cytogenetic risk status, and improved outcomes in patients with high-risk disease.
Fonseca:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Applied Biosciences: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Onyx/Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Binding Site: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Siegel:Celgene Corporation: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Merck: Speakers Bureau. Dimopoulos:Celgene: Honoraria; Onyx: Honoraria; Genesis: Honoraria; Janssen-Cilag: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Amgen: Honoraria. Spicka:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Masszi:Novartis: Consultancy; Janssen-Cilag: Consultancy; BMS: Consultancy; Takeda: Consultancy. Hájek:Janssen-Cilag: Honoraria; Celgene, Merck Sharp & Dohme: Consultancy, Honoraria. Rosiñol:Celgene: Honoraria; Janssen: Honoraria. Mateos:Janssen-Cilag: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy; Onyx: Consultancy. Wang:Celgene: Research Funding. Niesvizky:Celgene: Consultancy, Speakers Bureau. Oriol:Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Jakubowiak:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SkylineDx: Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi-Aventis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: institutional funding for support of clinical trial conduct, Speakers Bureau; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi-Aventis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SkylineDx: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Palumbo:Celgene, Millennium Pharmaceuticals, Amgen, Bristol-Myers Squibb, Genmab, Janssen-Cilag, Onyx Pharmaceuticals: Consultancy, Honoraria; Novartis, Sanofi Aventis: Honoraria. Bensinger:Onyx: Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acetylon: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium: Research Funding. Kukreti:Lundbeck: Honoraria; Ortho: Honoraria; Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Tonda:Onyx: Employment. Obreja:Amgen Inc: Employment. Moreau:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal