Abstract
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is an effective therapeutic option for adults with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) after the conventional cyclophosphamide and total-body irradiation (CY/TBI) regimen, but post-transplant relapse is still of high importance. High-dose cytarabine (HDCA) can be added to CY/TBI for an intensified regimen; however, its additional effects have not yet been completely elucidated.
METHODS
We conducted a cohort study to compare the prognosis of HDCA/CY/TBI and CY/TBI in HCT for AML/MDS, using a Japanese transplant registry database. Inclusion criteria are adult patients (aged 16 years or older) who underwent a single-unit cord blood transplantation (CBT) or bone marrow or peripheral blood stem cell transplantation (BMT/PBSCT) as a first HCT after CY/TBI (CY, 60 mg/kg for 2 days; TBI, 10-12 Gy divided into 4-6 fractions) or CY/TBI plus HDCA (HDCA/CY/TBI) between January 2000 and December 2012. HDCA was defined as cytarabine administration of 2 to 3 g/m2 twice a day for 2 to 3 days (total dose, 6-12 g/m2) before HCT.
Overall survival (OS) was calculated with the Kaplan-Meier method and compared using log-rank tests for each covariant related to pre-transplant patient characteristics. Factors with significance or borderline significance (p < 0.1) in the univariate analysis were subjected to a multivariate analysis using the Cox proportional hazards model. The Fine-Gray proportional hazards model was used in multivariate analyses for tumor-related mortality and non-relapse mortality (NRM).
Our protocol complied with the Declaration of Helsinki, and it was approved by the Registry Data Management Committee and by the Ethics Committee of Kyoto University, where the study was performed. Written informed consent was obtained from each patient at each institution.
RESULTS
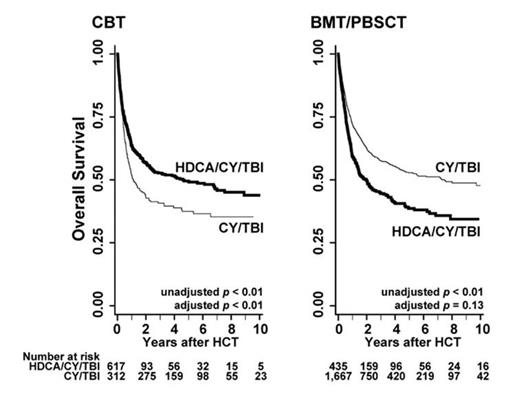
We analyzed data on 3,031 patients in total, composed of the HDCA/CY/TBI group (n = 1,052) and the CY/TBI group (n = 1,979) after CBT (n = 929) and BMT/PBSCT (n= 2,102). The median age was 38 years, and 86.1% of the patients had AML; high-risk disease was observed in 55.5% of the patients. In BMT/PBSCT, unrelated donors comprised 54.6%, and 63.9% of donors were HLA-matched. The median follow-up period after HCT was approximately 3.2 years. In CBT, OS was significantly superior in the HDCA/CY/TBI group compared to the CY/TBI group (adjusted hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.45-0.69; p < 0.01), while in BMT/PBSCT improvement of OS was not observed in the HDCA/CY/TBI group (HR, 1.14; p = 0.13) (refer to Figure). Tumor-related mortality was lower in HDCA/CY/TBI than CY/TBI after CBT (HR, 0.50; p < 0.01); in BMT/PBSCT, however, post-transplant relapse and tumor-related mortality were not suppressed by the addition of HDCA (HR, 0.89 and 0.90; p = 0.58 and 0.34, respectively). The incidence of grade II to IV acute graft-versus-host disease (GVHD) and chronic GVHD was significantly higher in the HDCA/CY/TBI group than the CY/TBI group after CBT (HR, 1.33 and 2.30; p = 0.02 and < 0.01, respectively), whereas there were no differences in BMT/PBSCT (HR, 0.95 and 1.04; p = 0.54 and 0.64, respectively). Incidence of infectious episodes (bacterial, viral, and fungal) showed no significant differences between regimens in both donor sources. Hemorrhagic cystitis and thrombotic microangiopathy increased in HDCA/CY/TBI compared to CY/TBI after BMT/PBSCT (HR, 1.47 and 1.60; p = 0.06 and 0.04, respectively), leading to significantly higher NRM in this group (HR, 1.48, p < 0.01). On the other hand, NRM was not increased by the addition of HDCA after CBT (HR, 0.94; p = 0.73).
CONCLUSION
This study is the first to show the differences between HDCA/CY/TBI and CY/TBI after HCT for AML/MDS using a large cohort. Superiority of HDCA/CY/TBI to CY/TBI in CBT was clearly indicated, while these merits were not applied to BMT/PBSCT. The higher incidence of GVHD and stronger graft-versus-leukemia (GVL) effects may be involved in these differences between donor sources (BMT/PBSCT compared to CBT). A large-scale prospective study is warranted to validate these results and to establish the suitable conditioning regimens including HDCA administration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal