Key Points
Frontline brentuximab vedotin monotherapy provided a 92% ORR and was generally well tolerated in elderly HL patients.
Abstract
Outcomes in older patients with Hodgkin lymphoma (HL) tend to be poor following conventional chemotherapy regimens. Treatment-related toxicity is significant and comorbidities often limit therapeutic options. This phase 2, open-label study evaluated the efficacy and safety of brentuximab vedotin, a CD30-directed antibody-drug conjugate, as frontline therapy in 27 HL patients aged ≥60 years. The objective response rate (ORR) was 92%, with 73% achieving complete remission. All patients achieved stable disease or better, and had decreased tumor volume following treatment. At the time of this analysis, the median duration of objective response for efficacy-evaluable patients (N = 26) was 9.1 months (range, 2.8 to 20.9+ months), median progression-free survival was 10.5 months (range, 2.6+ to 22.3+ months), and median overall survival had not been reached (range, 4.6+ to 24.9+ months). The observed adverse events (AEs) were generally consistent with the known safety profile of brentuximab vedotin. The most common AEs were peripheral sensory neuropathy (78%), fatigue (44%), and nausea (44%), and were ≤ grade 2 for most patients. The incidence of grade 3 peripheral neuropathy events was relatively high (30% overall), particularly among patients with the known risk factors of diabetes and/or hypothyroidism (46% vs 14% for those without). However, these risk factors were not associated with delayed time to resolution/improvement of peripheral neuropathy. Preliminary data showed no substantial age-related changes in brentuximab vedotin pharmacokinetics. Brentuximab vedotin monotherapy may provide a frontline treatment option for older patients who cannot tolerate conventional combination chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT01716806.
Introduction
Classical Hodgkin lymphoma (HL) is histopathologically defined by the presence of malignant Hodgkin Reed-Sternberg cells that express the CD30 surface antigen. The age at diagnosis has a bimodal distribution, with peaks between 15 to 34 and ≥55 years of age.1 Approximately 9190 patients were diagnosed with HL in the United States during 2014,2 and up to 20% of newly diagnosed HL patients are estimated to be aged ≥60 years.3-5
Outcomes among older patients with HL tend to be inferior to those of younger patients treated with standard chemotherapy regimens.5-8 The biology of HL in older patients may differ from that in younger patients, as manifested by more frequent mixed cellularity histology and Epstein–Barr virus positivity, which is associated with increased HL-specific mortality.9 Additionally, the presence of comorbidities, particularly cardiac dysfunction, may limit the administration of standard intensity anthracycline-containing regimens.10,11 Evens et al8 evaluated frontline treatment of HL with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) and Stanford V (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) regimens in older vs younger patients. Using a competing risks model to account for deaths due to other causes, the authors found that at 5 years, patients aged ≥60 years had significantly worse failure-free survival (48% vs 74% for younger patients; P = .002) and overall survival (OS; 58% vs 90% for younger patients; P < .0001). Among pooled ABVD and Stanford V treatment arms, the 5-year incidence of death in older patients was 21% due to HL progression, 9% due to treatment-related toxicity, and 12% due to other causes.8 In the first year after diagnosis, the incidence of death due to toxicity in older patients exceeded that due to HL progression. Because this patient population tolerates conventional initial therapy poorly, evaluation of novel less toxic treatment strategies is necessary.
Brentuximab vedotin (ADCETRIS) is a CD30-directed antibody-drug conjugate (ADC). After binding to CD30 on the tumor cell surface, preclinical data suggest that the primary mechanism of action is internalization of the ADC complex, leading to release of the microtubule-disrupting agent monomethyl auristatin E (MMAE), and subsequent cell-cycle arrest and apoptosis.12,13
In a pivotal phase 2 study of brentuximab vedotin in relapsed/refractory HL (median patient age, 31 years),14 the objective response rate (ORR) was 75%; 34% of patients attained a complete remission (CR). The median duration of objective response was 6.7 months, and 20.5 months for CR. The median OS was 40.5 months (range, 1.8 to 48.3+ months).15
In a retrospective evaluation of brentuximab vedotin monotherapy in patients ≥60 years with relapsed/refractory HL (median age, 66 years),16 the ORR was 56% and CR rate was 38%. The median duration of objective response had not been reached. The median OS was 12.4 months (range, 1.9 to 31.4 months), and the median progression-free survival (PFS) was 9 months (range, 1.9 to 23.3+ months). Brentuximab vedotin was well tolerated, and the number of cycles received and dose intensity were similar to those of younger patients (median age, 32 years).
Accordingly, the current phase 2, open-label evaluation of single-agent brentuximab vedotin was initiated to prospectively investigate the efficacy and safety of brentuximab vedotin as frontline therapy in HL patients aged ≥60 years. Additionally, since chronologic age alone does not capture the heterogeneity present within an elderly population, baseline geriatric assessment was conducted to more fully characterize the patients.
Patients and methods
Patient eligibility
Patients were aged ≥60 years with classical HL (ie, patients with nodular lymphocyte predominant HL [NLPHL] were excluded). Patients were treatment naïve and were either ineligible for frontline conventional combination treatment of HL (eg, ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone [BEACOPP]) in the investigator’s judgment or had declined the available chemotherapy options after being informed of the potential benefits and risks. Patients had fluorodeoxyglucose-avid disease by positron emission tomography (PET), measurable bi-dimensional disease ≥1.5 cm, and Eastern Cooperative Oncology Group (ECOG) performance status ≤3. Other inclusion criteria included absolute neutrophil count ≥1000/μL; platelets ≥50 K/μL; bilirubin ≤2× the upper limit of normal (ULN), and alanine aminotransferase/aspartate aminotransferase ≤3× ULN (or bilirubin ≤3× and aspartate aminotransferase/alanine aminotransferase ≤5× ULN if documented hepatic involvement with lymphoma). Patients could not have renal disease requiring ongoing dialysis. Baseline renal function was based on estimated creatinine clearance (CrCl) as determined using the Cockroft-Gault formula,17 and categorized as mild, moderate, or severe (CrCl >50 to 80 mL/min, 30 to 50 mL/min, or <30 mL/min, respectively).
Exclusion criteria included symptomatic neurologic disease compromising activities of daily living or requiring medications; history of progressive multifocal leukoencephalopathy; and active viral, bacterial, or fungal infection ≥ grade 3 within 2 weeks prior to the first dose of brentuximab vedotin.
The study was approved by the Institutional Review Board at each site and written informed consent was obtained from all patients prior to any study-specific procedures, in accordance with the Declaration of Helsinki.
Study design and treatment
This study describes the results of frontline brentuximab vedotin monotherapy in HL patients ≥60 years old; data were collected from October 2012 to March 2015, and follow-up continues for some patients.
The primary objective was to assess the ORR. Secondary objectives were to evaluate duration of response, CR rate, PFS, and resolution of B symptoms; safety and tolerability; and pharmacokinetics (PK).
Patients received 1.8 mg/kg of IV brentuximab vedotin every 3 weeks for up to 16 doses. Patients with clinical benefit per the investigator could, with consent of the medical monitor, continue treatment beyond 16 cycles until disease progression, unacceptable toxicity, or study closure. Patients with an estimated CrCl <30 mL/min were to receive 1.2 mg/kg brentuximab vedotin. Dose reduction to 1.2 mg/kg and treatment delay of up to 3 weeks were allowed depending on the type and severity of toxicity, including peripheral neuropathy. Supportive measures were provided throughout the study according to institutional standards.
Study assessments
Response was assessed by the investigators using the Revised Response Criteria for Malignant Lymphoma.18 Computed tomography (CT) and PET scans were performed at baseline, cycles 2 and 8, and end of treatment. Assessment with CT alone was performed at cycles 4 and 12. For patients continuing beyond 16 cycles, CT and PET were assessed at Cycle 16, then per institutional standard of care (at least every 6 cycles). Once CR was achieved, PET scans were no longer required. Patients who discontinued treatment without disease progression were restaged per standard of care (at least every 6 months) for the first 2 years, and annually thereafter.
Safety evaluations included adverse events (AEs) and laboratory abnormalities, and continued for ≥30 days following the last dose of brentuximab vedotin.
For PK evaluations, blood samples were collected pre-dose, at the end of infusion, and 24, 48, 168, and 336 hours post-infusion (Days 2, 3, 8, and 15) for cycle 1, and quantified as previously described.19
A baseline geriatric assessment was administered to evaluate physical function, comorbidities, nutritional status, cognition, psychological state, and social activity/support. The Blessed Orientation-Memory-Concentration (BOMC) test20 was included; the treating physician was notified if a patient scored >11, indicating cognitive impairment. The exploratory geriatric assessment used in this study incorporates validated tools known to predict morbidity and mortality in community-dwelling older adults.21 A similar assessment by the Cancer and Aging Research Group identified older patients most at risk for toxicity from chemotherapy.22
Statistical analysis
The primary end point was ORR. Secondary efficacy end points were the CR and disease control rates; durations of objective response, CR, and PFS; and B-symptom resolution rate. Efficacy was evaluated for all treated patients with classical HL histology who had a baseline and ≥1 post-baseline disease assessment or documented clinical progression of disease after receiving brentuximab vedotin. The ORR and CR rates and their exact confidence intervals (CIs) were calculated using the F distribution method.23 Durations of response, CR, and PFS were analyzed using Kaplan–Meier survival methodology.
An ORR ≥25% was considered to represent a threshold for clinical benefit, given that the patients may not have had other options for initial conventional therapy. The null hypothesis was that the ORR was ≤25%. With a sample size of 30 efficacy evaluable patients and an overall significance level of 0.1, observing ≥13 objective responses would allow rejection of the null hypothesis. Assuming a true ORR of 75%, the power was >90%.
Safety was evaluated for all patients treated with brentuximab vedotin. AEs were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.1; AEs and laboratory results were graded using the National Institutes of Health (NIH) National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
PK parameters (area under the concentration-time curve [AUC], maximum concentration [Cmax], and time of maximum concentration) were estimated by noncompartmental analysis using Phoenix WinNonlin 6.3 (Pharsight, Mountain View, CA), and summarized for patients who received 1.8 mg/kg brentuximab vedotin at cycle 1.
Results
Patients
Twenty-seven patients were treated with frontline brentuximab vedotin monotherapy at 16 clinical sites in the United States; 20 patients (74%) were from community-based sites. Demographic and baseline disease characteristics are presented in Table 1. The median age was 78 years (range, 64 to 92 years); 17 patients were aged ≥75 years and 5 were aged ≥85 years. The median percentage of CD30-positive malignant cells by immunohistochemistry was 90% (range, 20% to 100%); 2 patients had <60% positive cells. Most patients (63%) had stage III or IV disease.
Baseline demographics and disease characteristics
| . | N = 27 . |
|---|---|
| Age (y), median (min, max) | 78.0 (64, 92) |
| Age distribution, n (%) | |
| <65 y | 2 (7) |
| ≥65 and ≤74 y | 8 (30) |
| ≥75 and ≤84 y | 12 (44) |
| ≥85 y | 5 (19) |
| Gender, male, n (%) | 14 (52) |
| ECOG performance status, n (%) | |
| Grade 0/1 | 21 (78) |
| Grade 2/3 | 6 (22) |
| Karnofsky performance status, median (min, max) | 90 (60, 100) |
| Histologic subtype of HL | |
| Nodular sclerosis | 12 (44) |
| Mixed cellularity | 5 (19) |
| Lymphocyte-rich classical HL | 4 (15) |
| Lymphocyte-depleted classical HL | 1 (4) |
| Classical HL NOS | 4 (15) |
| Other (NLPHL)* | 1 (4) |
| Stage at diagnosis, n (%) | |
| Early stage I/II | 10 (37) |
| Late stage III/IV | 17 (63) |
| Percent CD30+ malignant cells, median (min, max) | 90 (20, 100) |
| B symptoms, n (%) | 6 (22) |
| Bulky disease, n (%) | 6 (22) |
| Extra-nodal involvement, n (%) | 14 (52) |
| Renal impairment category,† n (%) | |
| Unimpaired (CrCl >80 mL/min) | 8 (30) |
| Mild (CrCl >50 to ≤80 mL/min) | 7 (26) |
| Moderate (CrCl ≥30 to ≤50 mL/min) | 12 (44) |
| Cardiac ejection fraction, %, median (min, max) | 65 (49, 74) |
| . | N = 27 . |
|---|---|
| Age (y), median (min, max) | 78.0 (64, 92) |
| Age distribution, n (%) | |
| <65 y | 2 (7) |
| ≥65 and ≤74 y | 8 (30) |
| ≥75 and ≤84 y | 12 (44) |
| ≥85 y | 5 (19) |
| Gender, male, n (%) | 14 (52) |
| ECOG performance status, n (%) | |
| Grade 0/1 | 21 (78) |
| Grade 2/3 | 6 (22) |
| Karnofsky performance status, median (min, max) | 90 (60, 100) |
| Histologic subtype of HL | |
| Nodular sclerosis | 12 (44) |
| Mixed cellularity | 5 (19) |
| Lymphocyte-rich classical HL | 4 (15) |
| Lymphocyte-depleted classical HL | 1 (4) |
| Classical HL NOS | 4 (15) |
| Other (NLPHL)* | 1 (4) |
| Stage at diagnosis, n (%) | |
| Early stage I/II | 10 (37) |
| Late stage III/IV | 17 (63) |
| Percent CD30+ malignant cells, median (min, max) | 90 (20, 100) |
| B symptoms, n (%) | 6 (22) |
| Bulky disease, n (%) | 6 (22) |
| Extra-nodal involvement, n (%) | 14 (52) |
| Renal impairment category,† n (%) | |
| Unimpaired (CrCl >80 mL/min) | 8 (30) |
| Mild (CrCl >50 to ≤80 mL/min) | 7 (26) |
| Moderate (CrCl ≥30 to ≤50 mL/min) | 12 (44) |
| Cardiac ejection fraction, %, median (min, max) | 65 (49, 74) |
NOS, not otherwise specified.
One patient who received brentuximab vedotin was later determined to have NLPHL (protocol violation).
No patient had severe renal impairment (CrCl <30 mL/min).
Fourteen patients (52%) had been deemed ineligible for treatment with conventional multi-agent chemotherapy by the investigator; notably 10 patients (37%) with heart disease. Nineteen patients (70%) had mild or moderate renal impairment, including 2 patients (7%) with chronic renal disease. Other comorbidities that could limit the administration of standard chemotherapy were hypertension (18 patients, 67%), diabetes (7 patients, 26%), stroke (3 patients, 11%), peripheral vascular disorder (1 patient, 4%), and other malignancy (1 patient, 4%; myelofibrosis).
Baseline geriatric assessment results are summarized in Table 2 (for additional details, please see supplemental Tables 1 and 2 available on the Blood Web site). Twenty-two patients (81%) were impaired in at least 1 aspect. Eighteen patients (67%) reported being “limited a lot” for ≥1 physical activity,24 and 2 (7%) were completely dependent on others for ≥1 instrumental activity of daily living.25 In the preceding 6 months, 8 patients (30%) had fallen at least once and 9 patients (33%) had ≥10% unintentional weight loss. Fourteen patients (52%) reported having significant comorbidities. Seven patients (26%) had abnormal scores and 2 patients (7%) had cognitive or memory impairment based on the BOMC test.20 Few patients reported significant difficulty with social support.27
Baseline geriatric assessment results
| Area of assessment . | Tool for evaluation . | Definition of impairment . | N = 27, n (%) impaired . |
|---|---|---|---|
| Functional | Physical functioning24 * | “Limited a lot” for ≥1 activity† | 18 (67) |
| status | Instrumental activities of daily living25 ‡ | Completely dependent for ≥1 task† | 2 (7) |
| Number of falls in last 6 mo | ≥1 fall† | 8 (30) | |
| Timed “Up and Go”26 | >13.5 s to complete action§ | 13 (48) | |
| Comorbidities | Physical health25 ‡ | ≥3 comorbidities or ≥1 that significantly interfered with quality of life† | 14 (52) |
| Nutrition | Calculated % unintentional weight loss in the last 6 mo | ≥10% weight loss | 9 (33) |
| Cognitive function | BOMC test20 | >11 points (maximum 28)|| | 2 (7) |
| >6 points (above normal range) | 7 (26) | ||
| Social support | Social support survey27 * | ||
| Question: availability of help if confined to bed | Answer: “None or a little of the time”† | 2 (7) | |
| Question: availability of help with daily chores if ill | Answer: “None or a little of the time”† | 2 (7) |
| Area of assessment . | Tool for evaluation . | Definition of impairment . | N = 27, n (%) impaired . |
|---|---|---|---|
| Functional | Physical functioning24 * | “Limited a lot” for ≥1 activity† | 18 (67) |
| status | Instrumental activities of daily living25 ‡ | Completely dependent for ≥1 task† | 2 (7) |
| Number of falls in last 6 mo | ≥1 fall† | 8 (30) | |
| Timed “Up and Go”26 | >13.5 s to complete action§ | 13 (48) | |
| Comorbidities | Physical health25 ‡ | ≥3 comorbidities or ≥1 that significantly interfered with quality of life† | 14 (52) |
| Nutrition | Calculated % unintentional weight loss in the last 6 mo | ≥10% weight loss | 9 (33) |
| Cognitive function | BOMC test20 | >11 points (maximum 28)|| | 2 (7) |
| >6 points (above normal range) | 7 (26) | ||
| Social support | Social support survey27 * | ||
| Question: availability of help if confined to bed | Answer: “None or a little of the time”† | 2 (7) | |
| Question: availability of help with daily chores if ill | Answer: “None or a little of the time”† | 2 (7) |
Medical Outcomes Study.
Patient reported.
Older American Resources and Services subscale.
Patient action: stand up from chair, walk a distance of 3 meters, turn, walk back to chair and sit down.
Both patients scored 14 out of a maximum 28; the treating physician was notified.
Among the 18 patients with impaired physical function on geriatric assessment, 8 (44%) had preexisting peripheral neuropathy events based on medical history, compared with 1 of 9 patients (11%) without impaired physical function. However, preexisting neuropathy did not correlate with falls in the 6 months prior to study entry: 3 of 8 patients who had fallen (38%) had preexisting neuropathy, as did 6 of 19 patients (32%) without falls.
Efficacy
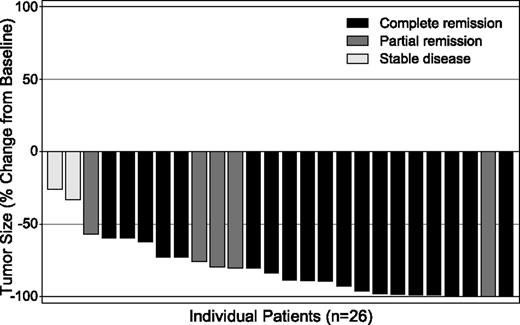
The ORR among the 26 efficacy-evaluable patients was 92% (Table 3). One treated patient was excluded from efficacy analyses due to an ineligible histology of NLPHL. Nineteen patients (73%) achieved a CR and 5 (19%) a partial remission (PR); the remaining 2 patients achieved stable disease. No consistent correlation was observed between response and improvement in ECOG status. Six patients had B symptoms at baseline that resolved during treatment. All evaluable patients had decreased tumor volume by CT scan measurement following treatment with brentuximab vedotin (Figure 1).
Best clinical response to frontline brentuximab vedotin monotherapy
| . | N = 26* . | ||
|---|---|---|---|
| . | n . | % . | 95% CI† . |
| ORR (CR + PR) | 24 | 92 | (74.9, 99.1) |
| Best clinical response | |||
| CR | 19 | 73 | (52.2, 88.4) |
| PR | 5 | 19 | — |
| SD | 2 | 8 | — |
| Disease control rate (CR + PR + SD) | 26 (100) | 100 | (86.9, 100) |
| . | N = 26* . | ||
|---|---|---|---|
| . | n . | % . | 95% CI† . |
| ORR (CR + PR) | 24 | 92 | (74.9, 99.1) |
| Best clinical response | |||
| CR | 19 | 73 | (52.2, 88.4) |
| PR | 5 | 19 | — |
| SD | 2 | 8 | — |
| Disease control rate (CR + PR + SD) | 26 (100) | 100 | (86.9, 100) |
SD, stable disease.
Efficacy-evaluable patients.
Two-sided, exact CI.
Maximum tumor size reduction from baseline. All 26 efficacy-evaluable patients achieved tumor reduction. Tumor size was assessed by measurement of index lesions on CT scans, whereas overall response (indicated by the color of the bars) was based on the Revised Response Criteria for Malignant Lymphoma,18 which incorporates both CT and PET scan results.
Maximum tumor size reduction from baseline. All 26 efficacy-evaluable patients achieved tumor reduction. Tumor size was assessed by measurement of index lesions on CT scans, whereas overall response (indicated by the color of the bars) was based on the Revised Response Criteria for Malignant Lymphoma,18 which incorporates both CT and PET scan results.
The median duration of objective response was 9.1 months (range, 2.8 to 20.9+ months) for all responders, 4.1 months (range, 3.9 to 10.3 months) for PR, and 9.2 months (range, 2.8 to 20.9+ months) for CR.
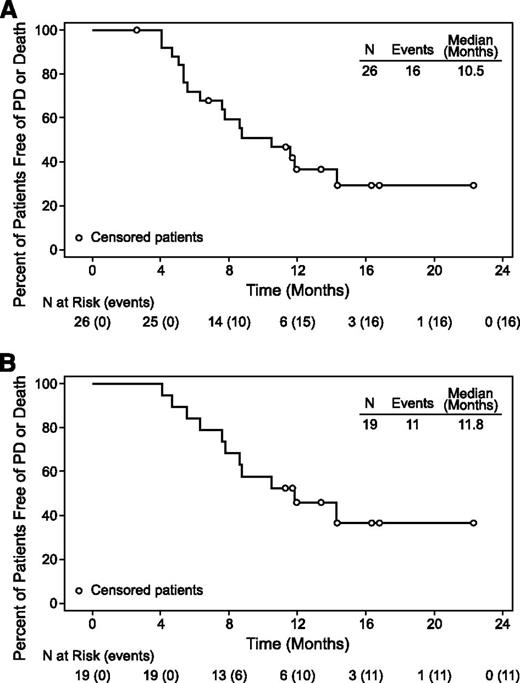
At the time of this analysis, the median observation time from first dose was 17.0 months (range, 4.6 to 24.9 months). The median PFS was 10.5 months (range, 2.6+ to 22.3+ months) for all efficacy-evaluable patients and 11.8 months (range, 4.1 to 22.3+ months) for CR (Figure 2). No pattern of baseline characteristics or treatment intensity appeared evident among the 6 patients with PFS >12 months (Table 4), who have maintained remissions for a median of 6.5 months off therapy (range, 1.0 to 12.2 months). The OS for all efficacy-evaluable patients ranged from 4.6+ to 24.9+ months and the median OS had not been reached.
PFS for all patients and for patients with CR. (A) Median PFS was 10.5 months (range, 2.6+ to 22.3+ months) in all efficacy-evaluable patients (N = 26). (B) Median PFS was 11.8 months (range, 4.1 to 22.3+ months) in patients with CR (n = 19). Censored patients are indicated by open circles (○).
PFS for all patients and for patients with CR. (A) Median PFS was 10.5 months (range, 2.6+ to 22.3+ months) in all efficacy-evaluable patients (N = 26). (B) Median PFS was 11.8 months (range, 4.1 to 22.3+ months) in patients with CR (n = 19). Censored patients are indicated by open circles (○).
Characteristics of patients with PFS greater than 12 months
| Age (y)/gender . | Histologic subtype of HL (stage) . | Percent CD30+ malignant cells . | Baseline SPD (cm2) . | Number of treatment cycles . | Number of dose delays (dose reduced to 1.2 mg/kg) . | Best clinical response (first observed) . | Reason for EOT . | Time off treatment (mo) . | PFS (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 71/F | Mixed cellularity (IIa) | 100 | 28.7 | 23 | 2 (cycle 10) | CR (cycle 2) | Investigator decision* | 2.7 | 22.3+ |
| 76/F | Mixed cellularity (IVb) | 20 | 12.9 | 16 | 0 (cycle 16) | CR (cycle 4) | AE (PSN) | 5.7 | 16.8+ |
| 64/F | Classical HL NOS (IIb) | 90 | 5.0 | 6† | 0 (no reduction) | CR (EOT) | AE (PSN) | 12.2 | 16.3+ |
| 84/M | Nodular sclerosis (IIIa) | 100 | 37.3 | 15 | 3 (cycle 6) | CR (cycle 2) | AE (PSN) | 1.0 | 14.3+ |
| 84/M | Classical HL NOS (IIIa) | 80 | 14.7 | 9 | 3 (cycle 4) | CR (cycle 2) | AE (PSN) | 7.4 | 14.3‡ |
| 78/M | Nodular sclerosis (IIa) | 90 | 64.4 | 5 | 0 (no reduction) | CR (EOT) | AE (orthostatic hypertension) | 9.8 | 13.4+ |
| Age (y)/gender . | Histologic subtype of HL (stage) . | Percent CD30+ malignant cells . | Baseline SPD (cm2) . | Number of treatment cycles . | Number of dose delays (dose reduced to 1.2 mg/kg) . | Best clinical response (first observed) . | Reason for EOT . | Time off treatment (mo) . | PFS (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 71/F | Mixed cellularity (IIa) | 100 | 28.7 | 23 | 2 (cycle 10) | CR (cycle 2) | Investigator decision* | 2.7 | 22.3+ |
| 76/F | Mixed cellularity (IVb) | 20 | 12.9 | 16 | 0 (cycle 16) | CR (cycle 4) | AE (PSN) | 5.7 | 16.8+ |
| 64/F | Classical HL NOS (IIb) | 90 | 5.0 | 6† | 0 (no reduction) | CR (EOT) | AE (PSN) | 12.2 | 16.3+ |
| 84/M | Nodular sclerosis (IIIa) | 100 | 37.3 | 15 | 3 (cycle 6) | CR (cycle 2) | AE (PSN) | 1.0 | 14.3+ |
| 84/M | Classical HL NOS (IIIa) | 80 | 14.7 | 9 | 3 (cycle 4) | CR (cycle 2) | AE (PSN) | 7.4 | 14.3‡ |
| 78/M | Nodular sclerosis (IIa) | 90 | 64.4 | 5 | 0 (no reduction) | CR (EOT) | AE (orthostatic hypertension) | 9.8 | 13.4+ |
EOT, end of treatment; PSN, peripheral sensory neuropathy; SPD, sum of the products of the largest diameters of up to 6 index lymph nodes or nodal masses being followed for response assessment.
No definite evidence of lymphoma after 23 cycles.
Consolidative radiation after EOT.
Death due to respiratory failure 8 months after the last dose of brentuximab vedotin; preexisting coronary artery disease, myelofibrosis, and macrocytic anemia.
Safety
Patients received a median of 8 cycles of brentuximab vedotin (range, 3 to 23 months), with 4 patients completing 16 cycles and 1 patient completing 23 cycles. Peripheral neuropathy events were the primary AEs leading to dose modifications. Fourteen patients (52%) had dose delays, typically 1 week (range, 1 to 3 weeks). Eleven (41%) had permanent dose reductions to 1.2 mg/kg.
All patients had at least one AE. The most commonly reported AE terms were peripheral sensory neuropathy (21 patients, 78%), fatigue (12 patients, 44%), and nausea (12 patients, 44%) (Table 5). These events were typically grade 1 or 2 in severity. Myelosuppression was minimal. Treatment-related anemia and neutropenia of grade 2 or 3 were reported for 2 patients each. No patient had thrombocytopenia or febrile neutropenia. Two patients received prophylactic pegfilgrastim during the study. No pulmonary AEs ≥ grade 3 was reported. There were no infusion-related or hypersensitivity reactions to brentuximab vedotin.
Treatment-emergent AEs occurring in at least 15% of patients treated with frontline brentuximab vedotin monotherapy
| . | N = 27 . | ||
|---|---|---|---|
| Event* . | Grade 1/2 n (%) . | Grade 3† n (%) . | Total n (%) . |
| Peripheral sensory neuropathy | 14 (52) | 7 (26) | 21 (78) |
| Fatigue | 12 (44) | 0 | 12 (44) |
| Nausea | 12 (44) | 0 | 12 (44) |
| Peripheral edema | 10 (37) | 0 | 10 (37) |
| Diarrhea | 9 (33) | 0 | 9 (33) |
| Decreased appetite | 8 (30) | 0 | 8 (30) |
| Constipation | 7 (26) | 0 | 7 (26) |
| Alopecia | 5 (19) | 0 | 5 (19) |
| Cough | 5 (19) | 0 | 5 (19) |
| Muscular weakness | 5 (19) | 0 | 5 (19) |
| Pruritus | 5 (19) | 0 | 5 (19) |
| Rash | 3 (11) | 2 (7) | 5 (19) |
| Urinary tract infection | 4 (15) | 1 (4) | 5 (19) |
| Vomiting | 5 (19) | 0 | 5 (19) |
| Deep vein thrombosis | 4 (15) | 0 | 4 (15) |
| Dizziness | 4 (15) | 0 | 4 (15) |
| Maculopapular rash | 3 (11) | 1 (4) | 4 (15) |
| Weight decreased | 4 (15) | 0 | 4 (15) |
| . | N = 27 . | ||
|---|---|---|---|
| Event* . | Grade 1/2 n (%) . | Grade 3† n (%) . | Total n (%) . |
| Peripheral sensory neuropathy | 14 (52) | 7 (26) | 21 (78) |
| Fatigue | 12 (44) | 0 | 12 (44) |
| Nausea | 12 (44) | 0 | 12 (44) |
| Peripheral edema | 10 (37) | 0 | 10 (37) |
| Diarrhea | 9 (33) | 0 | 9 (33) |
| Decreased appetite | 8 (30) | 0 | 8 (30) |
| Constipation | 7 (26) | 0 | 7 (26) |
| Alopecia | 5 (19) | 0 | 5 (19) |
| Cough | 5 (19) | 0 | 5 (19) |
| Muscular weakness | 5 (19) | 0 | 5 (19) |
| Pruritus | 5 (19) | 0 | 5 (19) |
| Rash | 3 (11) | 2 (7) | 5 (19) |
| Urinary tract infection | 4 (15) | 1 (4) | 5 (19) |
| Vomiting | 5 (19) | 0 | 5 (19) |
| Deep vein thrombosis | 4 (15) | 0 | 4 (15) |
| Dizziness | 4 (15) | 0 | 4 (15) |
| Maculopapular rash | 3 (11) | 1 (4) | 4 (15) |
| Weight decreased | 4 (15) | 0 | 4 (15) |
AEs were summarized using MedDRA version 15.1 and graded using the NIH National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
No grade 4 events were reported for these AE terms.
Only two grade 4 events, both considered unrelated to brentuximab vedotin, occurred during the study (hyperuricemia in 1 patient; drug hypersensitivity to anesthesia in another). Grade 3 AEs related to brentuximab vedotin were reported for 13 patients (48%). Related grade 3 AEs reported for more than 1 patient were peripheral sensory neuropathy (7 patients, 26%) and peripheral motor neuropathy and rash (2 patients; 7% each, respectively).
A standardized MedDRA query was used to evaluate a broad spectrum of peripheral neuropathy events. Twenty-four patients (89%) had treatment-emergent peripheral neuropathy events during the study; the events resolved or improved for 14 of those patients. Among the 10 patients with unresolved neuropathy, there was no consistent pattern of changes in ECOG performance status. Risk factors for neuropathy included diabetes and/or hypothyroidism (13 patients, 48%), monoclonal gammopathy of unknown significance (1 patient, 4%), and preexisting neuropathy (9 patients, 33%).
Eight patients (30%) had treatment-related grade 3 neuropathy events. Only 2 of the 9 patients with preexisting neuropathy at baseline (grade 1 or 2) developed grade 3 neuropathy after treatment. Among patients with diabetes and/or hypothyroidism (n = 13), the incidence of grade 3 peripheral neuropathy events (6 patients, 46%) was higher compared with those without these risk factors (n = 14; 2 patients, 14%). However, no association with these risk factors was noted in the time to the first grade 3 event (median of 16.6 weeks vs 15.4 for patients without risk factors), or time to resolution or improvement of any peripheral neuropathy event (median 9.8 weeks vs 8.9 for patients without risk factors).
At the time of this analysis, all patients had discontinued treatment; 18 (67%) remained on study in long-term follow-up. Reasons for treatment discontinuation were progressive disease after initial response (11 patients, 41%), AEs (11 patients, 41%), patient decision (3 patients, 11%), investigator decision (1 patient, 4%), and other non-AE reason (1 patient, 4%). AEs that led to treatment discontinuation were peripheral sensory neuropathy (8 patients, 30%), peripheral motor neuropathy (2 patients, 7%), and orthostatic hypotension (1 patient, 4%).
After discontinuation of brentuximab vedotin, 14 patients received subsequent lymphoma treatment, including 3 who received palliative radiation. The subsequent therapy most often received was doxorubicin, vinblastine, and dacarbazine (AVD; 5 patients). Four of the 15 patients deemed by the investigator to be ineligible at baseline for conventional standard full-dose, multi-agent chemotherapy received subsequent chemotherapy after discontinuing brentuximab vedotin: AVD (2 patients); gemcitabine, carboplatin, and dexamethasone (GCD; 1 patient); and single-agent gemcitabine (1 patient). The response and duration of response to subsequent therapies were not collected in this study.
Five deaths occurred after the end of the AE reporting period. Three deaths were related to HL progression. One was due to heart attack 6 months after the last dose of brentuximab vedotin in an 88-year-old patient with hypertension and hypercholesterolemia. One was due to respiratory failure 8 months after the last dose of brentuximab vedotin in an 84-year-old patient with preexisting coronary artery disease, myelofibrosis, and macrocytic anemia. Six patients (22%) had serious AEs. Three patients experienced treatment-related serious AEs (grade 1 pyrexia in one; grade 3 orthostatic hypotension in another; grade 3 asthenia, grade 2 deep vein thrombosis, and grade 2 rash in the third).
PK
Preliminary cycle 1 PK data were summarized for 26 patients who received 1.8 mg/kg IV brentuximab vedotin at cycle 1. Peak concentrations typically occurred at the end of infusion for the ADC and ∼2 days post-infusion for MMAE. The mean ADC AUC0-∞ and Cmax were 110 d•μg/mL and 44.4 μg/mL, respectively, and the mean MMAE AUC0-∞ and Cmax were 34.8 d•ng/mL and 5.06 ng/mL, respectively.
Discussion
Although the incidence of HL is elevated in individuals aged ≥65 years relative to younger patients28 and is expected to increase by 70% by 2030,29 few clinical trials specifically enroll older adults, who typically have more comorbidities and may be more susceptible to chemotherapy toxicities. Thus, this population continues to remain substantially under-represented in clinical trials.30-32
This is the first prospective study of brentuximab vedotin therapy in older patients with HL. Patients treated in the study were representative of the typical geriatric HL population and most (74%) were from community-based sites. Enrollment was restricted to patients aged ≥60 years and most patients (63%) were aged ≥75 years. Almost one-fourth of the patients had an ECOG performance status of 2 or 3, and approximately half would not have been offered conventional therapy (eg, ABVD or BEACOPP) due to age and/or comorbidities, underscoring the large unmet need for alternative options such as brentuximab vedotin therapy.
The ORR, the primary efficacy variable for this study, was 92%, exceeding the 25% ORR considered to represent a threshold for clinical benefit in this patient population. Nineteen patients (73%) achieved a CR, and all evaluable patients had decreased tumor volume following treatment. At the time of this analysis, the median duration of objective response for all efficacy-evaluable patients was 9.1 months (range, 2.8 to 20.9+ months) and the median PFS was 10.5 months (range, 2.6+ to 22.3+ months).
The 92% ORR achieved with brentuximab vedotin monotherapy compares favorably with recent reports in elderly patients with HL. Evens et al reported an ORR of 85% in 95 patients, 70% of whom received ABVD,7 and an ORR of 68% in 44 patients treated with ABVD or Stanford V in approximately equal proportions.8 Similarly, Stamatoullas et al reported an 84% ORR in 147 older HL patients treated with ABVD.33 The median PFS of 10.5 months in the current study is shorter than the observations of ∼3 years reported in the Evens studies.7,8 The Evens et al and Stamatoullas et al reports7,8,33 represent the limited data available describing outcomes in elderly HL patients following standard frontline combination therapy. However, the median ages of the patients in those reports were 65 to 68 years vs 78 years in the current study. Moreover, the retrospective design, and differences in assessment schedules and response criteria (ie, the inclusion of PET data) make direct comparison with the current study difficult.
The addition of dacarbazine or bendamustine to brentuximab vedotin, which may improve the durability of response, is currently being explored in separate cohorts of this study. The potential for combining brentuximab vedotin with bendamustine as salvage therapy in younger HL patients was demonstrated recently.34 Additionally, preclinical data suggest that dacarbazine and brentuximab vedotin could be synergistic (Julie A. McEarchern, Dana Kennedy, Renee McCormick, Timothy S. Lewis, Martha Anderson, Weiping Zeng, Eric L. Sievers, and Che-Leung Law; unpublished data, October 26, 2010).
The AEs observed in the older HL patients in this study were generally consistent with the known safety profile of brentuximab vedotin. Most patients (89%) experienced treatment-emergent peripheral neuropathy events. The incidence of grade 3 neuropathy events was relatively high (30%) and the median time to onset was 15.4 weeks. In contrast, in the pivotal phase 2 study of brentuximab vedotin in relapsed/refractory HL,14 11% of patients had grade 3 neuropathy events and the median time to onset was 38 weeks. The difference in frequency and severity of neuropathy may be due, at least in part, to the patients’ age (median age, 78 vs 31 years in the pivotal study) and the presence of comorbidities known to be associated with neuropathy, including diabetes and hypothyroidism. Preexisting neuropathy, however, did not appear to predispose the patients in this study to the development of grade 3 neuropathy. Management of neuropathy included dose delays and reductions to 1.2 mg/kg. Neuropathy events resolved or improved in 58% of the affected patients.
In comparison with historical data for conventional frontline chemotherapy for HL, the incidence of myelosuppression and pulmonary toxicity was substantially lower in this trial. No pulmonary AEs ≥ grade 3 were reported, whereas Evens et al7 reported a 32% incidence of bleomycin lung toxicity (with an associated mortality rate of 25%) among 95 patients, 70% of whom were treated with ABVD. Stamatoullas et al33 reported a 27% incidence of bleomycin lung toxicity (20% early; 7% late) among 147 ABVD-treated patients, leading the investigators to propose a reduction or omission of bleomycin from the regimen; 29% of deaths in that study were due to toxicity, including 14% due to pulmonary toxicity. Two patients (7%) in our study had treatment-related neutropenia, both ≤ grade 3; and no thrombocytopenia or febrile neutropenia occurred. In contrast, Repetto et al10 reported a higher risk of grade 4 neutropenia, neutropenia-related infection, and mortality in lymphoma patients aged ≥60 years treated in clinical trials of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or regimens with equivalent toxicity. Stamatoullous et al33 observed grade 3 or 4 thrombocytopenia or neutropenia in 24% of ABVD-treated patients.
Preliminary PK data suggest no substantial differences in exposures of ADC and MMAE across age groups in adults. Exposures in patients aged ≥60 years in the current trial were similar to those reported for a group of 14 patients with a median age of 39 years (range, 16 to 68 years; 2 patients ≥60 years).19 In the current trial, subgroup analyses of patients aged <75 years vs ≥75 years showed comparable exposures for both ADC and MMAE, and exposures in patients with mild and moderate renal impairment appeared comparable to those with normal renal function.35
This study includes one of the first prospective assessments of baseline geriatric function in HL patients and not only helps establish a benchmark for future studies, but also demonstrates the feasibility of collecting this information in real time. Additionally, the data may be useful in evaluating the similarity of elderly HL patients seen in clinical practice to this study population when considering brentuximab vedotin as a treatment option. Older individuals may be more fragile due to age-associated conditions such as functional losses, falls, and cognitive impairment.32 The geriatric assessment helped characterize the overall baseline status of the patients by evaluating factors other than diagnosis and age that might place an older patient at higher risk for morbidity and mortality from standard frontline intensive chemotherapy.36 This assessment revealed that 81% of patients were impaired in at least one aspect including physical performance problems, such as limited mobility (48%) and falls (30%), substantial comorbidities (52%), and poor nutrition (33% had ≥10% unintentional weight loss). These results illustrate the relatively fragile condition of the patients enrolled on this study. In future research, given that a large proportion of patients in this study had physical performance problems (eg, 67% had impaired physical function24 ), more extensive evaluation of side effects that may affect quality of life, and their impact on geriatric assessment domains, may be enlightening.
Although this study was open label and not randomized, and evaluated a relatively small number of patients, it demonstrated that brentuximab vedotin monotherapy may provide a frontline treatment option for older patients who are unable to tolerate conventional multi-agent chemotherapy. An attempt to improve response duration is currently being tested in additional treatment arms in this ongoing study, in which elderly HL patients receive a combination of brentuximab vedotin with either bendamustine or dacarbazine. Novel treatment approaches for elderly HL patients may offer promise for this fragile population.
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 7-10, 2013.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014.
Presented in abstract form at the annual meeting of the American Society for Clinical Pharmacology and Therapeutics, New Orleans, LA, March 3-7, 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jing Yang for PK analysis and Roberta Connelly for assistance in manuscript preparation, both sponsored by Seattle Genetics, Inc. Dr Supriya G. Mohile (University of Rochester Medical Center, Rochester, NY) acted as a consultant, and provided expertise on conducting the geriatric assessment and evaluation of the resulting data.
Direct funding for this study was provided by Seattle Genetics, Inc, through the joint financial support of Seattle Genetics, Inc and Takeda Pharmaceuticals International Co. R.C. was supported by the NIH National Cancer Institute (K12CA001727 and CCITLA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: A.F.-T., R.C., and J.W.F. contributed to the concept and design of the study; A.F.-T., B.H., J.G., R.C., G.O., R.V.B., R.E.B., J.P.S., and C.A.Y. contributed to data acquisition; A.F.-T., M.C.P.-W., and C.A.Y. wrote the manuscript, and along with Y.W., analyzed and interpreted the data; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: Seattle Genetics, Inc, provided research funding to the institutions of A.F.-T., B.H., J.G., R.C., G.O., R.V.B., R.E.B., J.W.F., J.P.S., R.C., and C.A.Y. have acted as consultants for Seattle Genetics, Inc. R.V.B. and R.C. have participated in a Seattle Genetics, Inc speakers’ bureau. R.V.B. received funds for travel expenses from Seattle Genetics, Inc. M.C.P.-W. and Y.W. are employees of and have equity ownership in Seattle Genetics, Inc.
Correspondence: Andres Forero-Torres, University of Alabama at Birmingham, NP 2510, 1530 3rd Avenue South, Birmingham, AL 35294-3300; e-mail: aforero@uab.edu.