Abstract
Monoclonal B lymphocytosis (MBL) is defined as the presence of a clonal B-cell population in the peripheral blood with fewer than 5 × 109/L B-cells and no other signs of a lymphoproliferative disorder. The majority of cases of MBL have the immunophenotype of chronic lymphocytic leukemia (CLL). MBL can be categorized as either low count or high count based on whether the B-cell count is above or below 0.5 × 109/L. Low-count MBL can be detected in ∼5% of adults over the age of 40 years when assessed using standard-sensitivity flow cytometry assays. A number of biological and genetic characteristics distinguish low-count from high-count MBL. Whereas low-count MBL rarely progresses to CLL, high-count MBL progresses to CLL requiring therapy at a rate of 1% to 2% per year. High-count MBL is distinguished from Rai 0 CLL based on whether the B-cell count is above or below 5 × 109/L. Although individuals with both high-count MBL and CLL Rai stage 0 are at increased risk of infections and second cancers, the risk of progression requiring treatment and the potential to shorten life expectancy are greater for CLL. This review highlights challenging questions regarding the classification, risk stratification, management, and supportive care of patients with MBL and CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonal lymphoproliferative disorder characterized by >5 × 109/L peripheral B-lymphocytes coexpressing CD5, CD19, and CD23 and a weak expression of CD20, CD79b, and surface immunoglobulin (sIg).1 When such a population is detected in enlarged lymph nodes of patients without peripheral lymphocytes, the term small lymphocytic lymphoma (SLL) is used, indicating a clinical variant of the same histopathological and molecular entity.2
The possibility of a precursor state to CLL was first identified in the early 1990s when a series of cross-sectional population-based studies was conducted in the United States to determine the health risks of living near hazardous waste sites.3,4 Using a 2-color panel (CD19 and CD5), 11 out of 1926 (0.6%) individuals older than 40 years were found to have a clonal population of CD5+CD19+ B cells, an immunophenotype classically associated with CLL. However, none of them met the diagnostic criteria for CLL or SLL. In particular, none had an absolute lymphocyte count (ALC) >5000/µL, as originally required by the diagnostic criteria for CLL.5 This phenomenon, later categorized monoclonal B-cell lymphocytosis (MBL), opened a new chapter in the field of B-cell lymphoproliferative disorders, suggesting that a precursor state of these lymphoid malignancies may occur at high prevalence in the general population.
Evaluation of lymphocytosis
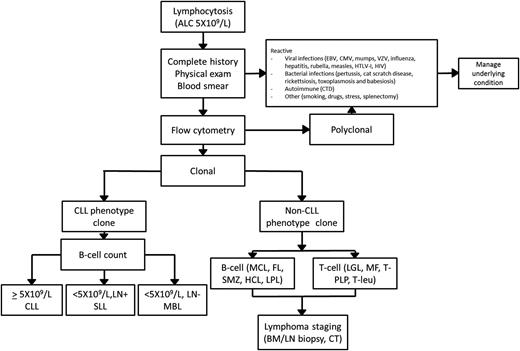
Lymphocytosis is a laboratory finding frequently encountered by the general internist and/or hematologist. An ALC ≥5 × 109/L has been suggested as the threshold in need of further investigation to identify infectious, autoimmune, or neoplastic etiology.6,7 A general approach to the workup of lymphocytosis is suggested in Figure 1.
General approach to the workup of lymphocytosis. BM, bone marrow; CMV, cytomegalovirus; CTD, connective tissue disease; EBV, Epstein-Barr virus; FL, follicular lymphoma; HCL, hairy cell leukemia; HTLV, human T-lymphotropic virus; LGL, large-granular leukemia; LN, lymph nodes; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MF, mycosis fungoides; T-PLP, T prolymphocytic leukemia; SMZ, splenic marginal zone lymphoma; T-leu, T-cell leukemia; VZV, varicella zoster virus.
General approach to the workup of lymphocytosis. BM, bone marrow; CMV, cytomegalovirus; CTD, connective tissue disease; EBV, Epstein-Barr virus; FL, follicular lymphoma; HCL, hairy cell leukemia; HTLV, human T-lymphotropic virus; LGL, large-granular leukemia; LN, lymph nodes; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MF, mycosis fungoides; T-PLP, T prolymphocytic leukemia; SMZ, splenic marginal zone lymphoma; T-leu, T-cell leukemia; VZV, varicella zoster virus.
A complete history and physical examination should represent the first step of such an evaluation, aimed at identifying causes of reactive (polyclonal) lymphocytosis. The most common cause of reactive lymphocytosis is viral infections, including hepatitis infection and HIV infection. Autoimmune conditions (particularly connective tissue diseases), smoking, hypersensitivity reactions, acute stress, and splenectomy can also induce polyclonal lymphocytosis.8,9
If the clinical and laboratory evaluation point toward a neoplastic origin, clonality should be evaluated through flow cytometry. A variety of clonal B-cell disorders can be identified based on surface protein markers with such analysis (Table 1). The management of clonal disorders of CLL phenotype is the focus of the remainder of this review. The detection of clonal B cells with a non-CLL phenotype (non-CLL MBL) or T-cell monoclonal lymphocytosis should warrant further testing, including computed tomography (CT) imaging, bone marrow biopsy, and molecular and genetic studies according to the suspected lymphoproliferative disorder.10,11
Immunophenotype of common clonal B-cell disorders
| . | CD5 . | CD19 . | CD20 . | CD23 . | CD10 . | CD103 . | Dual CD11c/22 . | sIg . | CD200 . | Genetic defects . |
|---|---|---|---|---|---|---|---|---|---|---|
| CLL | + | + | Dim | + | − | − | − | Dim | + | — |
| MCL* | + | + | Bright | Dim/− | − | − | − | Bright | − | t(11;14) |
| FL | − | + | + | +/− | +/− | − | − | + | − | t(14;18) |
| MZL† | − | + | Bright | − | − | − | + | + | − | 7q− |
| HCL | − | Bright | Bright | − | − | + | Bright | + | + | — |
| LPL‡ | +/− | + | + | +/− | − | − | − | Dim | + | — |
| . | CD5 . | CD19 . | CD20 . | CD23 . | CD10 . | CD103 . | Dual CD11c/22 . | sIg . | CD200 . | Genetic defects . |
|---|---|---|---|---|---|---|---|---|---|---|
| CLL | + | + | Dim | + | − | − | − | Dim | + | — |
| MCL* | + | + | Bright | Dim/− | − | − | − | Bright | − | t(11;14) |
| FL | − | + | + | +/− | +/− | − | − | + | − | t(14;18) |
| MZL† | − | + | Bright | − | − | − | + | + | − | 7q− |
| HCL | − | Bright | Bright | − | − | + | Bright | + | + | — |
| LPL‡ | +/− | + | + | +/− | − | − | − | Dim | + | — |
It is important to look at the flow cytometry histograms to determine the intensity of expression and whether the staining is “all, none, or partial.” The immunophenotype profile of classic CLL is dim sIg and dim CD20; CD5 and CD23 expression (not partial expression for either) is critical. Some degree of immunophenotype overlap among CLL, marginal zone lymphoma, and lymphoplasmacytic lymphoma exists. If the diagnosis is uncertain based on peripheral blood flow cytometry, lymph node biopsy should be pursued.
CD23 is usually negative, but ∼20% of cases will have partial CD23 expression.
CD5 is positive in 10% to 20% of cases, and it may be bright or partial in these situations.
20% of cases are CD5+; CD23 is usually negative, but some cases will have partial CD23 expression.
FL, follicular lymphoma; HCL, hairy cell leukemia; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; sIg, surface immunoglobulin
Classifying patients with clonal cells of CLL phenotype
Definition and prevalence of MBL
In 2005, the International Familial CLL Consortium proposed the term “monoclonal B lymphocytosis” to define the presence of CLL-phenotype cells in the peripheral blood in the absence of other features of CLL or SLL. The initially proposed diagnostic criteria for CLL phenotype MBL are as follows12 :
Documentation of a clonal B-cell population in peripheral blood (light-chain restriction [abnormal κ/λ ratio or low sIg in >25% B cells] or heavy-chain monoclonal IGHV rearrangement).
B-cell count <5 × 109/L.
Presence of CLL phenotype (CD5, CD19, CD23 positive; CD20 and sIg dim [reduced]).
No evidence of lymphoma, infection, or autoimmune conditions.
This entity was later acknowledged by the International Working Group of CLL, which in 2008 revised the 1996 National Cancer Institute–sponsored Working Group diagnostic criteria for CLL and SLL to include MBL. These revisions also redefined the threshold to diagnose CLL based on the absolute B-lymphocyte count rather than the ALC.1 The prevalence of MBL observed in the initial reports was revised after subsequent studies using more sensitive flow cytometry evaluation strategies. Two population studies were conducted in Europe in 2002 and 2004, analyzing either individuals referred to the hospital for nonhematologic conditions or in primary health facilities for routine evaluation. Using a 4-color flow cytometry approach and acquiring 200 000 events, both studies reported a prevalence of MBL of ∼3.5% in individuals older than 40 years.13,14 A subsequent population study from Italy, employing a 5-color panel and 500 000 acquisitions, found a prevalence of 6.7% among healthy individuals older than 40 years.15 A more sensitive technique was finally used, with an 8-color panel and 5 000 000 acquisitions, and reported a prevalence of 12% in healthy subjects of the same age.16 These studies indicated that large increases in assay sensitivity resulted in only small increases in the prevalence of MBL, indicating that MBL is not a universal phenomenon and only impacts a subset of the population.17,18
Risk factors for developing MBL
The findings above imply that MBL is not a physiological event that occurs in all individuals with increasing age but a specific condition affecting select patients in whom at least some predisposing risk factors have been identified (Table 2). A genetic predisposition for MBL is suggested by family studies. CLL has one of the strongest inherited predispositions among lymphoid malignancies. Familial CLL is defined as a pedigree with at least 2 first-degree relatives with CLL and characterizes about one-tenth of patients with CLL. The prevalence of MBL among unaffected relatives in such familial pedigrees is two- to threefold higher (∼15% among individuals older than 40 years) than that of the general population.19-21 Relatives of patients with sporadic CLL also have an increased prevalence of MBL, with a prevalence of 15.6% among relatives older than 60 years.22 Single-nucleotide polymorphisms in over 20 normal genes have now been found to be associated with familial CLL.23,24 Of interest, early studies of MBL demonstrated that at least 6 of these single-nucleotide polymorphisms known to confer an increased risk of developing CLL also relate to the risk of developing MBL in a large case-control series, supporting the concept of a genetic basis for MBL.25
Risk factors for MBL onset and progression to CLL requiring therapy
| Risk factors for MBL onset . | Risk factors for MBL progression to CLL . |
|---|---|
| Family history of CLL | CD38 positivity |
| Genetic polymorphisms* | Unmutated IGHV |
| Age | Deletion 17p |
| Infections† | Elevated B-cell count |
| Risk factors for MBL onset . | Risk factors for MBL progression to CLL . |
|---|---|
| Family history of CLL | CD38 positivity |
| Genetic polymorphisms* | Unmutated IGHV |
| Age | Deletion 17p |
| Infections† | Elevated B-cell count |
More than 20 single-nucleotide polymorphisms associated with the development of CLL have been reported.110 At least 6 of these have also been confirmed as risk factors for MBL (rs17483466, rs13397985, rs757978, rs872071, rs2456449, and rs735665),25 whereas the association of the others with MBL remains under investigation.
Hepatitis C, pneumonia, influenza, cellulitis, upper respiratory infections, and herpes zoster.26-28
As outlined above, the frequency of MBL in the general population progressively increases with age. Although present in only 0.2% to 0.3% of individuals younger than 40 years, its frequency is 3.5% to 6.7% in those aged 40 to 60 years and 5% to 9% among individuals older than 60 years when assessed using standard-sensitivity assays.14,15 When assessed using the most sensitive assay techniques, this prevalence can be >20% in healthy individuals older than 60 years and as high as 75% in subjects older than 90 years.16 Several studies point to a link between MBL and infection. One recent study identified MBL in ∼30% of hepatitis C virus–infected patients.26 Population-based studies have also reported an increased risk of CLL among patients with pneumonia.26 Conversely, a lower incidence of MBL has been reported among patients vaccinated for influenza or pneumonia.27,28 Studies aimed to determine whether specific antigenic stimuli can lead to the development of MBL are ongoing and may shed light on its pathogenesis and natural history.
Evidence supporting current classification and remaining classification challenges
Although on a theoretic level, classifying patients based on the presence of peripheral blood ALC and/or enlarged lymph nodes may seem simple (eg, B-cell count <5 × 109/L and no nodes: MBL; B-cell count <5 × 109/L with enlarged nodes: SLL; B-cell count >5 × 109/L: CLL), the clinical circumstance is often far more nuanced, as will be discussed below.
Low- vs high-count MBL
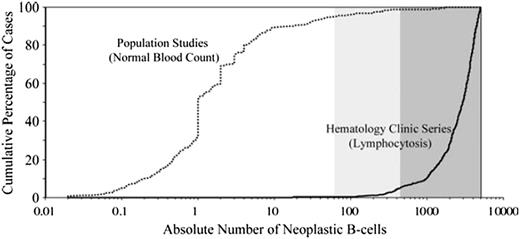
In 2010, a cumulative analysis of MBL series worldwide was performed and revealed the distribution of MBL cases followed a bimodal B-cell count distribution (Figure 2).29 Notable differences in the size of the clonal B-cell population between cases of MBL identified in population screening studies and clinical series were observed. Among cases identified in population screening studies, the overwhelming majority of patients had clonal B-cell counts between 0.1 and 10 clonal B cells/µL (median, 1/µL). In contrast, among cases identified in clinical cohorts (typically undergoing evaluation of low-level lymphocytosis), the clonal B-cell count was between 0.5 and 5 × 109/L (median, 2.9 × 109/L). Strikingly, little overlap was observed between the population and clinical cohorts, with very few cases lying in the middle. These results led to the descriptive segregation of MBL into 2 groups (high-count MBL and low-count MBL) based on the size of the B-cell clone (<0.5 × 109/L clonal B cells: low count MBL; ≥0.5 × 109/L clonal B cells: high count MBL).29-31
Distribution of clonal CLL-like B-cell count in published studies. Two main entities can be identified: low-count MBL, usually detected in general population studies, and high-count MBL, usually detected in series of patients referred for lymphocytosis. Reprinted with permission from Rawstron et al.29
Distribution of clonal CLL-like B-cell count in published studies. Two main entities can be identified: low-count MBL, usually detected in general population studies, and high-count MBL, usually detected in series of patients referred for lymphocytosis. Reprinted with permission from Rawstron et al.29
Biological characteristics of CLL, such as immunoglobulin repertoire, cytogenetic abnormalities, and, more recently, molecular mutations, were subsequently investigated in both low-count and high-count MBL, shedding light on their differential biology. Specific immunoglobulin gene rearrangements, frequently observed in patients with CLL, such as IGHV 4-34, 3-23, and 1-69, are underrepresented or absent in subjects with low-count MBL.32 By contrast, their frequency in high-count MBL is comparable to that observed in CLL.15 The frequency of IGHV-mutated cases is also significantly higher in individuals with low-count MBL than in subjects with high-count MBL or CLL.33 Moreover, whereas at least 25% of patients with CLL and 22% of subjects with high-count MBL demonstrate stereotyped complementarity determining region 3 sequences, these are present in <5% of individuals with low-count MBL.15,34 Cases of low-count MBL are also enriched with genetic abnormalities typically associated with more favorable prognosis in CLL, such as deletion 13q.35 By contrast, the distribution of genetic abnormalities as identified by fluorescence in situ hybridization (FISH) analysis in high-count MBL is comparable to that observed in CLL.16,36-38 High-throughput sequencing and new biological markers, such as noncoding RNA, may help to further characterize the biological differences of these 2 conditions.39,40 Novel mutations, such as NOTCH1 and SF3B1, described in 10% and 15% of patients with CLL, respectively, appear to be extremely rare in MBL, including high-count MBL.41,42 Additional gene mutations in patients with MBL continue to be discovered.43 Collectively, these findings suggest a potential stepwise evolution from high-count MBL to CLL through a progressive acquisition of high-risk genetic abnormalities but indicate that relatively few individuals with low-count MBL transition to high-count MBL.
High-count MBL vs CLL Rai stage 0
The threshold selected to distinguish high-count MBL from CLL (5 × 109 B cells/L) was arbitrary. Indeed, changing from an ALC-based criteria to a B-cell count–based criteria reclassified many patients who would have previously been considered Rai stage 0 CLL into the high-count MBL category.44,45 Initial analyses suggested, however, that the reclassification had clinical relevance, with different likelihoods of progression to require therapy for those in the high-count MBL and Rai 0 CLL categories.30,31,36 Nonetheless, as outlined above, many of the biological characteristics of high-count MBL are similar to Rai stage 0 CLL.46 These facts raised the question of whether high-count MBL should or should not remain an entity separate from Rai 0 CLL and, if a separate entity, what threshold should be used to segregate the 2 conditions. Because the designation of CLL and MBL are clinical diagnoses, a number of groups advocated that the distinction should be based on the clinical implications for patients, such as having an impact on survival. To answer this question, investigators from the Mayo Clinic evaluated what B-cell threshold best predicted overall survival among patients whose only manifestation of disease was a circulating clone of CLL phenotype (eg, no lymphadenopathy, organomegaly, or cytopenias). This analysis revealed that a B-cell threshold of between 10 and 11 × 109/L B cells was the best predictor of overall survival and predicted survival independent of other traditional molecular biomarkers.37 Three subsequent independent studies confirmed that a B-cell threshold of ∼10 × 109/L was the best predictor of survival.37,38,47,48 Notably, 10 × 109/L was also the B-cell threshold associated with changes in T-cell function, suggesting a potential biological underpinning for the association of this threshold with clinical outcomes.49 As can be easily intuited, higher B-cell counts are also associated with a shorter time to progression and a shorter time to first treatment.29,30
Nodal MBL
Accurate clinical assessment of lymphadenopathy is essential when evaluating individuals with MBL, because the presence of lymphadenopathy would suggest the diagnosis of SLL rather than MBL. Moreover, development of lymphadenopathy appears to be a common pattern of progression among individuals with MBL.29-31 This has raised the question of whether all individuals with MBL should be staged with a CT scan at time of diagnosis to rule out lymphadenopathy in nonpalpable lymph node regions. In one of the few studies evaluating this issue to date, 29 out of 62 individuals with clinically identified MBL (42%) had lymphadenopathy identified on CT imaging.50 Notably, however, after a median follow-up of 35 months, the rate of progression among cases of MBL with lymphadenopathy identified on CT imaging was only 6.9% (2/29 patients), which was no different than in cases of MBL without lymphadenopathy on CT imaging (rate of progression, 9%; 3/33 patients).50 These findings support the recommendation not to perform CT imaging in patients with MBL.51
It should also be noted that lymph node biopsy specimens used for the staging of other malignancies are often incidentally found to have a diffuse or focal infiltration of CLL-like cells in normal-sized or slightly enlarged lymph nodes.52 For example, up to 1.6% of patients undergoing sentinel lymph node biopsy for breast cancer show concomitant lymphoma, mostly with a CLL phenotype.53 How best to classify such patients (eg, SLL or MBL) when the circulating lymphocyte count is normal is unknown. Limited evidence suggests that patients in this circumstance with any lymph nodes ≥1.5 cm are at greater risk of progression/treatment.52 Based on this information, our current approach is to classify individuals with the incidental discovery of a CLL phenotype infiltrate in a normal-sized lymph node (eg, <1.5 cm), normal blood counts, and no other nodes >1.5 cm in size as nodal MBL and those with any enlarged lymph nodes (≥1.5 cm) as SLL. More studies evaluating how best to classify such patients are needed.
Other challenging situations and unanswered questions
Bone marrow involvement.
It is not uncommon to find a bone marrow infiltrate of CLL B cells in patients staged for other hematologic malignancies in the absence of other diagnostic criteria for either CLL or SLL. Of interest, most individuals with MBL who have a bone marrow biopsy at time of diagnosis have a concomitant bone marrow infiltration, with a median of 20% of marrow cellularity composed of CLL B cells.30,54 Although higher percentages of CLL infiltrate have been described,48,55 the percentage involvement is not clearly linked to the likelihood of clinical progression. Accordingly, it should be kept in mind that the diagnostic criteria for MBL do not include bone marrow features12 and, in the absence of other criteria, percent marrow infiltration does not change the diagnosis from MBL to CLL or SLL.1,56
Tissue involvement.
Clonal CLL-like cells can be detected in up to 0.4% of prostatic tissues at the time of prostatectomy57,58 and in 1.9% of liver biopsy specimens59 in the absence of meeting any other diagnostic criteria for CLL and SLL. Progression to first therapy has been only rarely reported in these cases, suggesting the concept of “tissue MBL.”56,60,61 Whether the coexistence of such a clone influences the outcome of the primary malignancy, as has been observed in CLL,62 is currently unknown. The extent of infiltration in such cases is also likely important, where extensive tissue replacement or infiltration is more consistent with a diagnosis of SLL.
Autoimmune disease.
According to the original guidelines, the presence of a concomitant autoimmune process excludes a diagnosis of MBL and was considered CLL.12 It has also long been recognized, however, that very small CLL-like clones can be found in individuals with immune thrombocytopenia and that many such patients may never develop any clinical manifestations of a lymphoproliferative disorder.63 In other patients with this profile, immune thrombocytopenia can occur many years before patients meet the criteria for CLL.63 How to best categorize patients with autoimmune conditions and very small clonal B-cell populations in blood or bone marrow remains challenging. Clinically, our approach is to consider individuals with autoimmune cytopenia who have very small B-cell clones (eg, normal lymphocyte counts and no lymphadenopathy) as “MBL with autoimmune cytopenia” rather than classifying them as CLL. Guidelines may need to be revised as new clinical/biological evidence becomes available.
Stem cell and blood donor issues.
Allogeneic stem cell transplantation (SCT) is an appropriate therapeutic option for selected high-risk patients with CLL,64,65 with matched related donors being the preferred source of stem cells. As outlined previously, MBL is more frequent in first-degree relatives of patients with both familial and sporadic CLL, raising the question of whether screening for MBL should be pursued in potential related stem cell donors. Indeed cases of MBL transfer from the donor to recipient during SCT have been described from both related and unrelated donors.66-68
Similar questions related to blood donation have also arisen. In 2007, studies using low-sensitivity flow cytometry indicated a prevalence of MBL of 0.14% in blood-bank donors.69 More recent studies using higher-sensitivity assays indicate a prevalence of MBL in blood donors of 7.1%.70 These findings are notable, given historical epidemiologic studies suggesting that blood transfusion is associated with an increased risk of developing CLL.71,72 Subsequent studies, however, refuted this finding, and the association in some historic studies may have been related to transfusion-associated hepatitis C.73 At present, there is no consensus as to whether routine screening for MBL among SCT donors should be pursued. Our approach is to screen all related donors with flow cytometry, independent of whether or not lymphocytosis is present, to identify cases of occult MBL.
Clinical implications and management of MBL
Clinical implications of low-count MBL
In 2009, using prediagnostic samples from the Prostate, Lung, Colon and Ovary Cancer Screening Trial study, investigators demonstrated that MBL could be observed in the peripheral blood of virtually all patients later diagnosed with CLL.74 This demonstrated for the first time that MBL is a precursor to CLL and that at least some cases progress. No information on the size of the B-cell clone was available in this study. However, based on the percent of lymphocytes that were clonal B cells, the vast majority of cases likely represented high-count MBL.
A few studies have prospectively examined the natural history of low-count MBL.75,76 The most robust data to date come from a prospective study of 1779 healthy adults from the Val Borbera valley in Northern Italy, of whom 138 had low-count MBL on screening (96 CLL-phenotype MBL, 21 atypical CLL-phenotype MBL, 20 CD5-negative–phenotype MBL). After a median follow-up of 34 months, a small clonal B-cell population persisted in 90% of those with CLL-phenotype MBL, but no patients progressed to CLL, SLL, or other lymphoid malignancy.65 Life expectancy for subjects with low-count MBL was also no different than in the general population.76 This evidence supports the current consensus that low-count MBL is at low risk of progression, has no clear clinical implications, and requires no specific clinical follow-up.51
Clinical implications of high-count MBL
A number of studies evaluating the natural history of high-count MBL indicate that the risk of progression to CLL or SLL requiring treatment is between 1% and 2% per year.30,36-38,51,77-79 As a consequence, an annual complete blood count and periodic lymph node examination are advised for individuals with high-count MBL.51
As a group, the overall survival of individuals with high-count MBL does not differ significantly from age- and sex-matched controls in the general population.38,80,81 A number of preliminary studies have suggested, however, that the prognostic parameters used in CLL (eg, IGHV mutational status, FISH, and CD38) have utility for stratifying outcome among individuals with high-count MBL30,31,78 and may identify a subset in whom the presence of MBL impacts overall survival.80
In contrast to individuals with low-count MBL,76 subjects with high-count MBL appear to have a significantly higher risk of hospitalization due to serious infections82 as well as a higher risk of hematologic83,84 and nonhematologic cancers.62,85 Given the prevalence of MBL, these findings could have public health implications, and more research in this area is needed.
Finally, although studies indicate that the diagnosis of CLL can precipitate substantial anxiety and adversely impact quality of life, data regarding the impact of an MBL diagnosis on quality of life are lacking.86
Clinical implications and management of early-stage CLL and SLL
Prognosis
Patients with CLL have a substantial shorter life expectancy compared with age- and sex-matched populations.87 A number of basic demographic and clinical characteristics, such as age, sex, and performance status, along with Rai and Binet staging systems, have been the foundation of risk stratification for the last 40 years.88,89 Although useful, most patients with CLL are now diagnosed with early-stage disease, where these parameters do not predict outcome for individual patients. Over the last 2 decades, a number of molecular biomarkers have been found to predict shorter survival. Inferior outcomes have been reported for patients with unmutated IGHV,90 positive CD38, ZAP70, or CD49,91-93 elevated serum β-2 microglobulin levels,94 and unfavorable genetic abnormalities such as deletion 11q and 17p on FISH testing95 or TP53 mutations on sequencing. The recent studies using next-generation sequencing have also identified a number of recurrent mutations such as NOTCH1, SF3B1, DDX3X, and ATM associated with clinical outcome.96
Several recent efforts have attempted to integrate the results of multiple prognostic markers into a single risk score.97,98 Although useful at the population level, most of these tools are not sufficiently robust to predict the outcome of individual CLL patients.
A comprehensive prognostic model was recently developed by the German CLL Study group. After analysis of 23 different prognostic markers in a cohort of ∼2000 patients participating in prospective clinical trials, the investigators identified 8 factors with independent prognostic value: age, sex, performance status, IGHV mutational status, FISH (deletion 17p13, deletion 11q23), serum β-2 microglobulin, and serum thymidine kinase. Each item was assigned a weighted score based on its hazard ratio for survival in the multivariate analysis. These values were then summed into a single risk score, which stratified patients into 4 risk groups with median 5-year survivals ranging from 95% to 18%.99 The prognostic index was then validated in an independent cohort of 700 newly diagnosed patients and was the first prognostic tool with sufficient accuracy to allow prediction of outcome in individual patients. Although a significant step forward, the cohorts in which this index was developed and validated are younger than the majority of real-world CLL patients. The index also did not account for the impact of comorbid health conditions, and incorporation of this information into future prognostic tools may further improve accuracy.
Building from the results of these prior efforts, a collaborative, international effort to develop an International Prognostic Index for patients with CLL is currently ongoing.
Indications for treatment
Phase 3 trials evaluating the benefit of early administration of chlorambucil compared with observation for asymptomatic early-stage CLL indicated a lack of clinical benefit for early treatment and established observation as the standard of care for early stage patients.100 According to the 2008 guidelines, the acceptable indications for treatment in patients with CLL are anemia (hemoglobin <11 g/dL) or thrombocytopenia (platelet count <100 × 109/L), progressive or symptomatic lymphadenopathy or organomegaly, and severe constitutional symptoms.1 Several recent studies have assessed the role of early treatment of select high-risk patients. Recent phase 3 trials comparing fludarabine to observation101 or fludarabine, cyclophosphamide, and rituximab to observation102 in early-stage patients at high risk of progression have again found no advantage of early treatment. These studies have further solidified active monitoring as the standard of care for asymptomatic early-stage patients outside of clinical trials regardless of the findings of prognostic testing. Whether the recent introduction of highly active, targeted, oral small-molecule inhibitors with a generally favorable toxicity profile (eg, ibrutinib and idelalisib) will change this paradigm is unknown. The German CLL Study Group is currently conducting a phase 3 study comparing ibrutinib with observation in patients with early-stage CLL at high-risk for progression. This study will provide important insights regarding the value of such an approach.
Supportive care
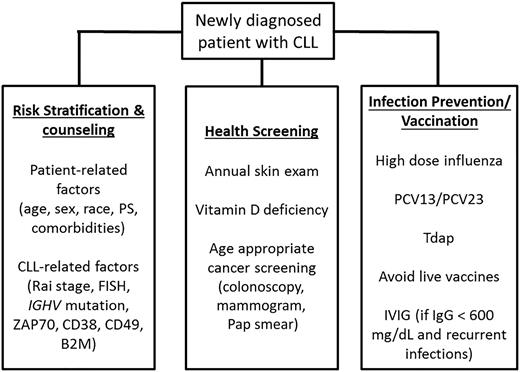
Patients with early-stage disease are at risk of a number of CLL-related complications, including nonhematologic cancers and infection. Second cancers, particularly skin cancers, are more frequent among CLL patients than in the general population.62,85 In addition to increased incidence, skin cancers may behave more aggressively in patients with CLL.103 Patients with CLL should be counseled regarding use of sun block and protective clothing and should undergo an annual skin examination. Sun avoidance can increase the risk of vitamin D deficiency, a condition that already affects 40% to 50% of the general US population. Given this fact and the association between vitamin D deficiency and CLL progression in several studies,104,105 it is reasonable to periodically assess vitamin D levels in patients with CLL. Given their apparent increased risk of other nonhematologic cancers, patients with CLL and high-count MBL should also adhere to age-appropriate cancer screening, such as colonoscopy, mammograms, and cervical cancer screening. Given their increased risk of infection, it is recommended that patients with CLL be kept up to date with influenza, pneumococcal,106 and tetanus, diphtheria, acellular pertussis vaccines despite the fact they are less likely to mount an immune response to vaccines (Figure 3). Live attenuated vaccines should be avoided.107
Management of early-stage CLL and SLL. B2M, β-2-microglobulin; IgG, immunoglobulin G; IVIG, intravenous immunoglobulins; MX, mammogram; PCV, pneumococcus vaccine; PS, performance status; Tdap, tetanus diphtheria, acellular pertussis.
Management of early-stage CLL and SLL. B2M, β-2-microglobulin; IgG, immunoglobulin G; IVIG, intravenous immunoglobulins; MX, mammogram; PCV, pneumococcus vaccine; PS, performance status; Tdap, tetanus diphtheria, acellular pertussis.
Finally, it must be noted that a family history of CLL is a strong risk factor for the development of CLL.108 In particular, first-degree relatives have a risk of developing CLL that is 2 to 7 times greater than the general population.109 This obviously represents a stressor among relatives of CLL patients, particularly in reference to children. Although guidelines about surveillance and counseling do not exist, periodic complete blood count and physical examination may be advised. Prospective observational studies may provide further hints in the future.
Acknowledgments
T.S. is a Clinical Scholar of the Leukemia and Lymphoma Society.
Authorship
Contribution: P.S. and T.D.S. conceived the study and wrote the paper.
Conflict-of-interest disclosure: T.D.S. has received research support from Genentech, Pharmacyclics/Janssen, Celgene, GlaxoSmithKline, Cephalon, Hospira, and Polyphenon E International. P.S. declares no competing financial interests.
Correspondence: Tait D. Shanafelt, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: shanafelt.tait@mayo.edu.