Abstract
Although once primarily recognized for its roles in hemostasis and thrombosis, the platelet has been increasingly recognized as a multipurpose cell. Indeed, circulating platelets have the ability to influence a wide range of seemingly unrelated pathophysiologic events. Here, we highlight some of the notable observations that link platelets to inflammation, reinforcing the platelet’s origin from a lower vertebrate cell type with both hemostatic and immunologic roles. In addition, we consider the relevance of platelets in cancer biology by focusing on the hallmarks of cancer and the ways platelets can influence multistep development of tumors. Beyond its traditional role in hemostasis and thrombosis, the platelet’s involvement in the interplay between hemostasis, thrombosis, inflammation, and cancer is likely complex, yet extremely important in each disease process. The existence of animal models of platelet dysfunction and currently used antiplatelet therapies provide a framework for understanding mechanistic insights into a wide range of pathophysiologic events. Thus, the basic scientist studying platelet function can think beyond the traditional hemostasis and thrombosis paradigms, while the practicing hematologist must appreciate platelet relevance in a wide range of disease processes.
Introduction
Thrombosis, inflammation, and cancer are interrelated, and circulating blood platelets are one cellular element common to each process. Although the relevance of platelets in the pathophysiology of thrombosis is well established, their contributions to inflammatory pathways and cancer are less defined and appear to be complex and multifaceted. Indeed, the paradigms of hemostasis and thrombosis, where a temporal sequence of events starts with an unactivated platelet recognizing a surface or being stimulated by an agonist and leads to an activated platelet, have dynamic implications for platelet phenotype and function. Similar changes are likely to have dramatic consequences for the platelet’s influence on both inflammation and cancer, and this highlights the likely complexity of platelet involvement in each disease process.
In 1863, Rudolf Virchow hypothesized that cancer could arise from sites of chronic inflammation, hypothesizing these sites could sustain proliferation.1 In fact, cancer has often been referred to as a wound that never heals, hijacking the body’s natural ability to fight infection and produce a proproliferative environment to sustain healing and tissue remodeling. We now know that cell proliferation alone is not sufficient to lead to or support transformation, but the risk of cellular transformation can be enhanced by cell proliferation in an environment rich in inflammatory cells, growth factors, activated stroma, and factors that promote DNA damage. Indeed, today it is widely accepted that there is a causal relationship between inflammation, innate immunity, and cancer development. To this end, the platelet’s natural role in wound healing is poised to be hijacked by these pathophysiologic events.
In this review, we highlight examples where the platelet interfaces with the inflammatory process. Then, in consideration of Virchow’s hypothesis, we will examine the hallmarks of cancer from the perspective of the blood platelet. Indeed, there have been remarkable molecular insights into the platelet’s relevance in thrombosis, inflammation, and cancer. However, going forward, these pathologic events can no longer be considered functional silos because the broader consideration of multifactorial events and their consequences will likely shape future innovations.
Platelets and the immune system
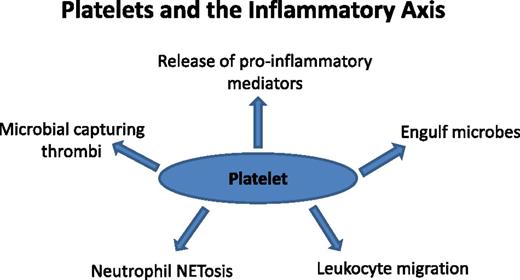
It is presumed that platelets and leukocytes share a common ancestral cell, the thrombocyte, facilitating both hemostatic and immune roles in lower vertebrates, such as fish and birds.2 Therefore, it comes as no surprise that platelets so frequently pervade immunologic functions.3 Although not strictly appointed within the inflammatory pathway, the platelet can be viewed as an extension of the cellular immune system.4-8 Recent evidence places the platelet in the middle of diverse inflammatory processes that influence normal leukocyte biology and inflammatory signals (Figure 1).
Platelets and the inflammatory axis. Shown and discussed in the text are circulating platelet properties that influence the inflammatory axis. Long studied for roles in hemostasis and thrombosis, platelets interact with granulocytes, vessel walls, and pathogens, positioning them to modulate the inflammatory response via both anti-inflammatory and proinflammatory mechanisms. Future studies must consider the state of platelet activation and how this affects the inflammatory response. Antiplatelet therapies traditionally considered only for cardiovascular disease treatment also can be evaluated for their effects on inflammation.
Platelets and the inflammatory axis. Shown and discussed in the text are circulating platelet properties that influence the inflammatory axis. Long studied for roles in hemostasis and thrombosis, platelets interact with granulocytes, vessel walls, and pathogens, positioning them to modulate the inflammatory response via both anti-inflammatory and proinflammatory mechanisms. Future studies must consider the state of platelet activation and how this affects the inflammatory response. Antiplatelet therapies traditionally considered only for cardiovascular disease treatment also can be evaluated for their effects on inflammation.
A number of key observations support the notion of the platelet as an immune cell. The platelet’s expression of Toll-like receptors (TLRs) indicates a capacity to directly engage microbial pathogens similar to leukocytes. Only a fraction of the platelet population is needed to maintain adequate hemostasis, suggesting that the excessive numbers allow platelets to act as major vascular surveyors of blood for recognizing foreign particles.9
Emerging evidence indicates the relevance of platelets in development of cardiovascular disease. Chronic inflammation is a primary factor in events leading to atherosclerosis. It has been documented that platelets adhere to von Willebrand factor (VWF) bound to endothelial cells, and this interaction elicits tethering and rolling of leukocytes on the endothelial surface.10 Thus, platelets not only bring leukocytes to a site where inflammation potentially leads to atherosclerosis but also contain stores of proinflammatory mediators, such as thromboxanes and CD40 ligand.11,12 Proteins of the complement system have a reciprocal relationship with platelets: platelets activate the complement system, and proteins of the complement system activate platelets.13 In experimental models, hyperlipidemia induces platelet recruitment to the endothelial layer, and crucial molecular players are VWF, glycoprotein Ib-IX, and P-selectin,14,15 although the role of glycoprotein Ib-IX is not without some controversy.16 Here, we again find an emerging role for familiar players from the platelet paradigm of hemostasis and thrombosis.
When platelet TLRs detect microbial species, platelet activation is instigated, wherein the cell degranulates and releases a myriad of proinflammatory mediators.17,18 The release of granule content and surface shedding results in an estimated 300 proteins and biomolecules being released into the proximal vasculature.19 CD154, also known as CD40 ligand, is a potent secretory molecule that elicits lymphocyte activation, and it has been the feature of numerous studies implicating its relevance for potentiating the adaptive immune response.20 Platelets are the primary source of soluble CD154 released after agonist-driven platelet activation.21,22 Furthermore, platelets secrete antimicrobial proteins (thrombocidins), reactive oxygen species, and lytic enzymes in conjunction with other granule cargo to further augment clearance of the immune insult.6
The ability of platelets to recognize and release a plethora of cytokines and chemokines in response to microbial invasion is merely one of the many facets of its arsenal to boost immunity. Platelets have exhibited an innate ability to engulf foreign particles such as human immunodeficiency virus bodies and Staphylococcus aureus cells into subcellular compartments.23,24 This internalization on activation localizes the microbes within the open canalicular system; although not entirely understood, this rudimentary ability does not appear to give rise to microbial degradation or any form of phagocytosis. Thus, the platelet acts as a storage cell for a plethora of bioactive molecules and lytic enzymes while maintaining a capacity to consume invaders, indicating that the platelet may in fact descend from an ancient granulocyte.25
Alhough the platelet is capable of directly attacking microbial insults, it also aids primary immune cells in the clearance of pathogens. As described in the context of atherogenesis, platelets can adhere to an activated/inflamed endothelium due to the upregulation of endothelial adhesion molecules. Likewise, platelets also associate with leukocytes in the circulation and, by adhering to the endothelium, succeed in tethering white cells to the vascular wall.26 This function has a physiologic counterpart to atherosclerosis because increasing the ability of leukocytes to form an interface with the vasculature bolsters the ability of these cells to migrate within infected tissues.27 By enhancing the ability of leukocyte migration, platelets aid in the clearance of an infection by boosting the number of white cells recruited to the target site.
The interaction between platelets and Kupffer cells in the liver appears to contribute to clearance of both bacterial cells and platelets during infection.24,28,29 Interestingly, TLRs aren’t the only platelet receptors capable of interacting with microbes; surface membrane glycoproteins, such as integrin αIIbβ3, glycoprotein Ib-IX, and FcγRIIa, all have been implicated in forming an interface with bacterial cells.18,30-32 Bacterial adhesion via these platelet receptors leads to platelet activation and release of secondary mediators, including platelet factor 4, to create a positive feedback activation mechanism.33 Studies using glycoprotein Ib-IX–deficient mouse platelets have suggested that the platelet glycoprotein Ib–IX/VWF axis supports the platelet–Kupffer cell interaction.24
The role of neutrophils in bacterial clearance has demonstrated these cells are capable of jettisoning nuclear content into the vasculature to ensnare migratory pathogens.34 The resultant web-like networks of chromatin, histones, and degradative enzymes known as neutrophil extracellular traps (NETs) capture circulating microbes and thus limit their ability to colonize alternate sites.35,36 Both in vitro and in vivo studies have illustrated that activated platelets adhering to neutrophils can initiate NET formation, or NETosis.37,38 Interestingly, experiments performed with the TLR4 agonist lipopolysaccharides (LPS), showed that, although capable of activating neutrophils, LPS alone is not potent enough to induce NET formation. However, when platelets are included, LPS administration is capable of eliciting NETosis, albeit this requires concentrations higher than those normally required to induce neutrophil activation.
Typically, formation of intravascular thrombi is regarded as a negative feature of platelet function; however, newer reports connect this feature to a physiologically relevant immune response. Microbial dissemination through the circulation increases the severity of infection. In the wake of microbial penetrance, a number of pathways initiate thrombogenesis in tandem with the inflammatory response.39 Controlled induction of thrombus formation within select vascular sites allows construction of a scaffolding network to ensnare microbial particles in a manner that parallels NETs. Although a thrombus is not traditionally considered a component of the immune response, evidence suggests that the clotting pathway activated in collaboration with inflammation is intended to serve a physiologic purpose. These “immunothrombi” are the result of several activating events converging on the players of hemostasis.
The very NETs that platelets help produce also have a reciprocal function. The negatively charged nucleobases of the NETs are capable of initiating the contact pathway of coagulation.40,41 The contact-dependent pathway raises circulating thrombin concentrations, which, in turn, increase platelet activation. Furthermore, NETs serve as a platform for the docking and activation of platelets.42 Direct engagement of microbes by platelets also contributes to platelet stimulation and thrombosis. An immunity-initiated thrombus not only serves as a dense network to trap migrating microbes but also acts as a foundation for tethering leukocytes, antimicrobial proteins, and lytic enzymes. By localizing the microbe with antimicrobial proponents, the immunothrombus serves as a focal point for microbial clearance.
Formation of additional platelet–leukocyte interfaces permits platelets to modulate the immune response. As previously mentioned, platelets can bolster the adaptive immune response by releasing CD154; moreover, they are capable of raising specific CD8+ T-cell clones by expressing antigen in the context of major histocompatibility complex I.43 Although more platelet endeavors within the immune cascade have yet to be uncovered, the current body of knowledge largely agrees with the notion of platelets as an extension of the immune system.
Platelet microparticles and inflammation
Platelet microparticles (membrane-bound fragments of platelet released on stimulation) have been linked to the chronic inflammation that produces rheumatoid arthritis.4,44 Specifically, in mouse models of rheumatoid arthritis, release of platelet microparticles elicits further inflammatory effector functions from synoviocytes to amplify synovitis in the disease. The microparticle release is mediated by platelet surface receptor glycoprotein VI, which complexes with the FcR-γ chain on the platelet surface. The best-characterized ligand of glycoprotein VI is collagen, and this binding presumably signals via immunoreceptor tyrosine-based activation motifs within the cytoplasmic tail of FcR-γ.45 The relevance of platelet microparticles in other inflammatory states has not yet been characterized.
Platelets and cancer
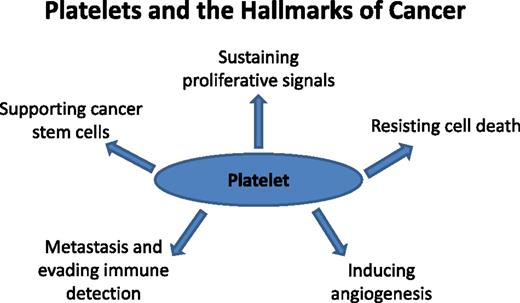
Cancer is a disease by which multiple complex changes within the tumor cell of origin and within the microenvironment fuel a “perfect storm” for disease progression and dissemination. In 2000, Hanahan and Weinberg defined 6 hallmarks of cancer: (1) self-sufficiency in growth signals, (2) insensitivity to growth-inhibitory signals, (3) resisting cell death, (4) limitless replicative potential, (5) sustained angiogenesis, and (6) metastasis.46 In 2011, the list was updated with addition of other essential characteristics, such as cellular and microenvironment alterations necessary for malignant transformation, dysregulation of cell energetics, avoidance of immune destruction, genomic instability, and tumor-promoting inflammation.47 Indeed, many of these hallmarks resemble the inflamed state, placing the platelet within an interface that links thrombosis, inflammation, and cancer (Figure 2).48
Platelets and the hallmarks of cancer. The original hallmarks of cancer included 6 biologic capabilities acquired during the complex development of tumors. Highlighted and discussed in the text are circulating platelet properties that contribute to some of the hallmarks. Thus, the platelet can be viewed as a normal cell contributing to the hallmark traits and influencing the TME. Antiplatelet therapies in the realm of cancer development and progression represent a future direction likely to impact patient prognosis and outcome.
Platelets and the hallmarks of cancer. The original hallmarks of cancer included 6 biologic capabilities acquired during the complex development of tumors. Highlighted and discussed in the text are circulating platelet properties that contribute to some of the hallmarks. Thus, the platelet can be viewed as a normal cell contributing to the hallmark traits and influencing the TME. Antiplatelet therapies in the realm of cancer development and progression represent a future direction likely to impact patient prognosis and outcome.
More than 45 years ago, it was established that thrombocytopenic mice are protected against metastasis.49 Since then, extensive data have supported the relevance of platelets in the progression of cancer.48 An appreciation for the relationship between blood coagulation and cancer started in the 1860s, when French physician Armand Trousseau observed that increased incidence of venous thrombosis and/or blood hypercoagulability was associated with certain cancers (Trousseau’s syndrome).50 Later, Dr Trousseau observed a procoagulant state within his own blood that preceded his death from pancreatic cancer. In the 20th century, the molecular basis of Trousseau’s syndrome was established with a direct demonstration of tumor cell-induced platelet aggregation.51,52 Subsequent work corroborated these seminal findings in a number of experimental models, each implicating a wide range of platelet receptors.53-57 The pathophysiologic influence of circulating platelets on aspects of tumorigenesis is varied and substantial, suggesting platelet therapies typically reserved for cardiovascular disease may have profound implications in cancer.
Sustaining proliferative signals
Tumorigenesis is a multistep process requiring concerted changes in both tumor cells and the tumor microenvironment (TME). It has often been equated to a wound that doesn’t heal, with sustained tissue proliferation and remodeling and not balanced with reciprocal apoptosis. For initiation and progression of disease, tumor cells require constant growth signals or the ability to produce these growth signals themselves. Many tumor cells acquire activating mutations in growth-sustaining signaling cascades, but these signals also can be received from the TME. Janowska-Wieczorek et al58 showed that platelet-derived microparticles stimulate mitogen-activated protein kinases in lung carcinoma cell lines and increase cell proliferation. Further, incubating A549 lung carcinoma cells with the microparticles led to increased expression of matrix metalloproteinases (MMPs) and increased invasion through matrigel. Platelets and their releasates can activate the same pathways that are activated through oncogenic mutations.
Resisting cell death
Proliferation and apoptosis are carefully orchestrated during tissue remodeling and inflammation. Tissues and cells are endowed with intrinsic regulatory programs to control aberrant proliferation, inducing programs of cell death. These intrinsic programs prevent unregulated cell growth and allow proper healing. Tumor cells must develop or find mechanisms to circumvent these intrinsic programs to sustain their proliferative capacity, and they must coordinate extrinsic programs to safeguard their survival. Recent studies demonstrated that stromal cells can extrinsically assist neoplastic cells to evade apoptosis.59 Platelets induce MMP-9 expression and activation in colon and breast cancer cell lines, leading to increased remodeling of extracellular matrix, release of growth factors from the extracellular matrix, and relief of cell-cell contacts, all of which decrease apoptotic signals.59 Platelets and platelet lysates reduce apoptosis of leukemia cell lines and primary leukemic blasts induced by mitochondria-damaging agents.60 Contents of platelet microparticles inhibited intrinsic apoptosis through mitochondrial uncoupling, independent of autophagy.60 These are some of the first studies to extend the antiapoptotic role of platelets beyond solid tumors. The results highlight the need for future studies to investigate the platelet’s roles in both hematopoietic and solid tumor malignancies and in disrupting cellular energetics and metabolism related to apoptosis.
Resistance to chemotherapy and to molecularly targeted therapies is a major obstacle to the successful treatment and management of cancer. Tumor cells develop resistance-promoting responses, including altered expression of integrins, activation of oncogenic signaling by soluble factors such as cytokines and growth factors, and resistance to apoptotic signals. The growth factor-rich microenvironment created by platelet degranulation and activation supports proliferation and is antiapoptotic, with thrombocytosis increasing chemoresistance and thrombocytopenia improving chemotherapy efficacy in murine models. In a murine model of breast cancer, induction of thrombocytopenia by platelet-depleting antibodies increased the efficacy of paclitaxel therapy.61 This effect was mediated by increased delivery of the chemotherapeutic drug to the tumor site, likely through increased tumor vascular permeability and increased tumor-specific hemorrhaging, which was not observed in other organs.
In a cohort of patients with ovarian cancer that was enriched for those with recurrent disease, elevated platelet counts correlated with decreased overall survival and resistance to chemotherapy.62,63 The study further demonstrated that platelet transfusion resulted in significantly greater tumor weight in nude mice harboring A2780 tumors than in untreated mice; this effect was completely reversed by pretreating the platelets with aspirin before transfusion.63 Further, treating tumor-bearing mice with platelet-depleting antibodies alone decreased tumor weight, similar to the effects of treatment with docetaxel alone. Treatment with both platelet-depleting antibodies and docetaxel further reduced tumor weight.63 Infusion with platelets during docetaxel therapy suppressed the efficacy of chemotherapy with no reduction in tumor weight. In vivo reduction of platelet counts reduced tumor growth to a similar extent as chemotherapy, and platelet reinfusion significantly reduced the efficacy of chemotherapy. These studies raise important clinical questions about the necessity for platelet replacement in thrombocytopenic cancer patients, indicating the possibility that platelet infusion may “feed” the tumor and decrease chemotherapeutic efficacy. Future studies are needed to define the roles of platelets in tumor growth and to assess the potential adjuvant roles of platelets during therapeutic intervention.
Inducing angiogenesis
Tumor cells proliferate at alarming rates, necessitating neovascularization to support adequate blood supply for supplying necessary nutrients, removing waste, and oxygenating the tumor. Historically, tumor vascularization was thought to be primarily regulated by tumor-derived proangiogenic factors; however, it is now clear that the TME and stromal cells significantly contribute to the neovascularization that occurs during tumor progression and that this vascular network provides a highway for disseminating tumors to distant sites. Platelets have the ability to deliver multiple proangiogenic factors to the tumor, as well as the ability to stimulate expression of proangiogenic factors by the tumor cell.58 Platelets have long been identified as a major source of vascular endothelial growth factor (VEGF),64,65 platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF), each promoting tumor growth.66,67 Activation of platelets and release of platelet microparticles leads to the release of a variety of proangiogenic factors, including VEGF, PDGF, FGF, and MMPs. Relevant to this release is the reported increase in the stored platelet content of VEGF, platelet factor 4, and PDGF of patients with colorectal cancer.68 Increased levels of platelet microparticles are found in the plasma of patients with both solid tumors and hematologic malignancies.69 In those with gastric cancer, the highest circulating levels of platelet microparticles were found in individuals with stage IV disease and were significantly correlated with metastatic disease.70 In vitro and in vivo studies demonstrated that platelet microparticles can promote proliferation and survival of endothelial cells, as well as vascularization in both healthy and diseased states.71,72
Activating invasion and metastasis and evading immune detection
Metastasis remains the biggest challenge to improving cancer prognoses and is the leading cause of cancer-associated mortality. Stromal cells provide the tumor cell with an ability to evade the immune system, recognize a premetastatic niche, and grow at a distant site; each highlights potential interplay among circulating tumor cells and all cells of the vasculature, including platelets. Data have suggested that a mechanism linking the platelet to metastasis is a platelet “cloak” that surrounds the tumor cell and protects it from immune surveillance.73 The ability of platelets to protect tumor cells in circulation from the normal immune response, or natural killer cells, is likely to significantly contribute to the metastatic process.73,74 Further, this platelet cloak protects tumor cells in circulation from extreme shear forces encountered in the vascular, preventing mechanical damage to the cells. The numerous receptors on the surfaces of platelets may help “dock” the cloaked tumor cells to the vascular endothelium and facilitate extravasation at a distant site. Releasates and direct interactions between platelets and tumor cells can contribute to sustaining growth and enhancing the ability of the tumor cells to migrate and colonize distant sites. Tumor cells can be primed by platelets while in circulation, or even in vitro, to induce a mesenchymal invasive phenotype and promote metastatic seeding in the lungs, mediated by transforming growth factor β signaling.59
Platelets also play a role in osteolytic bone metastasis in breast cancer. Boucharaba et al75 reported that platelet-derived lysophosphatidic acid (LPA) can support and stimulate metastatic breast cancer cells. MDA-BO2 breast cancer cells facilitate platelet aggregation and release of LPA, which has a potent mitogenic effect upon MDA-BO2 cells. Further, LPA stimulates the release of interleukin-6 and interleukin-8 from MDA-BO2 cells, which is hypothesized to stimulate osteoclasts in the bone marrow, leading to bone destruction and further supporting metastatic growth. Additional work is needed to determine whether platelet release of LPA stimulates metastasis in other cancer models and to identify the role of LPA in stimulating tumor cell cytokine release to enhance metastatic potential at distant metastatic sites. These findings highlight the multifactorial signaling networks that platelets are able to stimulate in the primary TME and at distant sites.
Supporting cancer stem cells
Although not a hallmark of cancer, support of accessory cells, particularly cancer stem cells, is crucial to the support of tumorigenesis and colonization at distant sites. It has become well established that tumors are comprised of a heterogeneous mix of cells, including a subpopulation of cells with self-renewing capacity. Stromal cells, including platelets, are important in supporting this subpopulation within the tumor. Labelle et al59 found that platelets cocultured with Ep5 breast carcinoma cells for 24 hours induced a cancer stem cell gene signature in the Ep5 cells. They further demonstrated that platelets promoted both the transforming growth factor β and nuclear factor κB pathways, inducing an epithelial-to-mesenchymal transition in breast and colon carcinoma cell lines, promoting a metastatic phenotype. High platelet count is associated with increased mortality in a variety of cancers, including malignant mesothelioma; gynecological malignancies; and lung, renal, gastric, colorectal, and breast cancers.62,76-83 We should seek to discern the roles played by platelets in stimulating different cellular compartments within the tumor, which has the potential to expand therapeutic use of cardiovascular inhibitors in oncology.
Cancer prevention and aspirin
In the last 2 years, interest has been renewed in cancer prevention mediated by daily use of aspirin, a controversial topic spanning several decades.84 Extensive use of aspirin for its cardiovascular benefits and inhibition of platelet function has resulted in significant datasets that now can be used to examine other benefits of aspirin. Although the antimetastatic properties of aspirin were first described in the 1970s based on animal models of experimental metastasis,85 other studies reported no such effect.86 However, a recent retrospective analysis from large patient datasets supports the anticancer effect of aspirin.87-90 Thus, the topic has once again gained interest, and major questions surround the molecular basis of the benefit and the role played by of the platelet.91
Aspirin directly inhibits cellular cyclooxygenase enzymes COX-1 and COX-2 and the production of prostaglandins. However, expression of COX-1 and/or COX-2 varies widely among cells. For example, endothelial cells and tumor cells express COX-2, but platelets express COX-1,92 which is the major contributor to aspirin’s side effect of bleeding. A significant gap in our understanding of aspirin’s anticancer effect lies in defining how much of the effect is dependent on platelets and how much is due to cyclooxygenase inhibition in other cell types. Virtually every step in the breast cancer metastatic process can be linked to normal platelet function.48 Determining the mechanism of action of aspirin in breast cancer and identifying the specific cells targeted by aspirin will allow development of therapeutic protocols that could mitigate the risks associated with prolonged aspirin therapy. The degree of aspirin’s protective action that depends on COX-1 vs COX-2 is unknown and was presented as one of the National Cancer Institute’s Provocative Questions for 2013.
Despite preclinical data and some positive clinical trials, the therapeutic strategy of antiplatelet drugs has not received significant attention from the cancer biology community. This may be due to the fact that antiplatelet drugs are not cytotoxic and therefore are ignored by the oncology community or to the mixed results of clinical trials that lead to the abandonment of these therapeutic strategies. However, this might also reflect a lack of communication and collaboration between the platelet and cancer biology fields. For too long, cancer biology has been tumor centric; however, with increased emphasis on the TME and the role of stromal cells in tumor initiation, progression, and metastasis, cancer biologists are reevaluating the roles of nontumor cells in carcinogenesis, including the roles of platelets. The widespread use of antiplatelet therapies emphasizes the importance for understanding mechanistic insights into the progression of cancer.
Concluding remarks
Inflammation is a process initiated by the host in response to injury. A multifactorial network of chemicals and cells is recruited to the site of injury to heal the tissue. Similarly, during tumorigenesis, many of these same processes are hijacked to promote cancer initiation and progression. In the ideal scenario, these mechanisms will act rapidly, and a normal state of homeostasis within the microenvironment will be attained. We attempted to present literature to highlight how these processes are used physiologically during the inflammatory process and when these same processes can become pathologic and support transformation and cancer progression. It is clear that additional studies are needed to bridge the fields of thrombosis and cancer and to elucidate mechanisms by which the platelet can promote and sustain tumorigenesis. Historically, thrombosis was viewed as an unfortunate consequence of malignancy, but emerging evidence strongly supports the fact that the platelet actively supports multiple stages of the tumorigenic process.
Although the platelet remains a long-term therapeutic target for preventing cardiovascular disease, how such treatment impacts other disease processes is relevant also to understanding molecular pathogenesis and to guiding patient treatment. Retrospective analyses of large populations taking daily aspirin have revealed an aspirin-dependent preventive effect for some cancers. Thus, new questions are emerging about the platelet-dependent mechanisms in cancer, which may also be relevant to chronic inflammation. Understanding the relevance and overlap of platelet function in each of these disease processes is likely to be informative on the risks and benefits of antiplatelet therapies and also should provide the practicing clinician with knowledge to facilitate active dialogue with patients on the risks and benefits of individual therapies.
Being associated with inflammatory events and cancer progression places the platelet within a myriad of complex pathophysiologic events.93 However, the well-defined paradigm of the platelet’s roles in hemostasis and thrombosis is likely to provide key insights into platelet responses in these intricately related disease processes. The goal would be to use the plethora of animal models and antiplatelet agents already available to further define platelet-mediated overlap among diseases not strictly associated with hemostasis and thrombosis. Future challenges will be to translate mechanistic findings from animal models to the course of human disease.
Caution will be needed as we move forward because some have recently reported marked differences between the mouse and human inflammatory responses.94 However, that study remains controversial, with others concluding that mouse models are representative of human inflammatory diseases.95 Indeed, many paradigms have translated well between species. In the case of platelets, the majority of thrombotic mechanisms are similar in mice and humans.96 Armed with decades of important platelet-dependent insights, the future is bright for broadening an appreciation of the platelet’s influence on many hematologic disorders.
Acknowledgments
The editorial assistance of Peggy Brenner, ELS, is acknowledged.
This work was supported by National Institutes of Health, Heart, Lung and Blood Institute grants HL50545 and AR61991 (to J.W.) and an American Heart Association predoctoral fellowship (to A.C.).
Authorship
Contribution: A.C. and J.W. wrote and edited the inflammation portion; and A.T.F. and J.W. wrote and edited the cancer portion.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerry Ware, Slot 505, 4301 West Markham St, Little Rock, AR 72205; e-mail: jware@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal