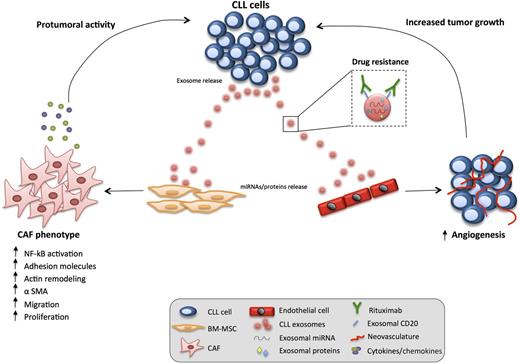
In this issue of Blood, Paggetti et al present novel findings that chronic lymphocytic leukemia (CLL)-derived exosomes and their molecular cargo are actively transferred to stromal cells that reside in the lymphoid tumor microenvironment (TME), promoting the reprogramming of these cells into cancer-associated fibroblasts (CAFs).1
CLL-derived exosomes have a paracrine effect on stromal cells residing in the TME. The transfer of exosomal cargoes (miRNA and proteins) to target cells (bone marrow, BM-MSCs, and endothelial cells) induces an inflammatory CAF phenotype in these cells that assume an aberrant stimulatory, protumoral role (increased angiogenesis, release of pro-survival chemokines/cytokines). In parallel, CLL-derived exosomes can act as decoy receptors for rituximab, thus representing a potential drug escape mechanism in the TME. BM, bone marrow; NK-κB, nuclear factor κB.
CLL-derived exosomes have a paracrine effect on stromal cells residing in the TME. The transfer of exosomal cargoes (miRNA and proteins) to target cells (bone marrow, BM-MSCs, and endothelial cells) induces an inflammatory CAF phenotype in these cells that assume an aberrant stimulatory, protumoral role (increased angiogenesis, release of pro-survival chemokines/cytokines). In parallel, CLL-derived exosomes can act as decoy receptors for rituximab, thus representing a potential drug escape mechanism in the TME. BM, bone marrow; NK-κB, nuclear factor κB.
CLL is characterized by an accumulation of monoclonal CD5+ mature B cells in lymphoid tissues and the peripheral blood. Clonal expansion and invasive migration typically cause the lymph nodes and bone marrow to become infiltrated with tumor. Recent progress in understanding the genetic landscape of lymphoid malignancies must also be coupled with research on the TME. Accumulating evidence is showing that bidirectional communication between cancer cells and their microenvironment is critical for tumor growth, but also profoundly affects therapeutic response. Malignant cells engage in novel associations/interdependencies with stromal cells including fibroblasts (mesenchymal stem cells [MSCs]), endothelial cells, and immune cells that provide crucial contributions to the licensing of tumor progression (survival, proliferation) and immune evasion.2 Many of the examples of heterotypic signaling studied to date involve classical paracrine signaling loops of cytokines or growth factors and their receptors. Although these signaling mechanisms are key mediators of cell-cell communication within the TME, more recently, exosome shedding has emerged as another mode of intercellular signaling. Exosomes are nanometer-sized endocytic vesicles manufactured within multivesicular endosomes and released into the extracellular compartment by many types of cells. Their biogenesis/release is enhanced when cells are stimulated—under stress or in a diseased environment. Transfer of exosomes and their cargo, which includes proteins, messenger RNA, and microRNAs (miRNAs), from cancer cells to other TME cell types has been the subject of intense studies in solid cancers,3 but understudied in lymphoid neoplasia. In this work, Paggetti et al1 provide comprehensive molecular analysis of exosomes derived from CLL cells and, in particular, functional data supporting a novel capacity of these extracellular vesicles to modulate the TME by reprogramming previously healthy stromal cells into CAFs.
The study uses a robust protocol to isolate exosomes from the supernatant of human CLL cells (MEC1 and primary samples). Elegant studies show that tumor-derived exosomes are rapidly taken up by stromal cells, including MSCs, endothelial cells, and myeloid cells (but not CLL cells). Interestingly, this later finding may contrast with microvesicles, a different type of vesicle arising from the outward budding of the plasma membrane, with data suggesting integration into CLL cells.4 Paggetti et al show that active exosome intercellular transfer requires specific functional surface proteins on exosomes (tetraspanins, integrins) and the presence of heparan sulfate proteoglycans on the surface of target cells (that CLL cells lack). The authors perform small RNA sequencing and mass spectrometry analysis of CLL exosomes to reveal predominantly regulatory RNAs, miscellaneous RNAs, and mature miRNAs enriched in CLL exosomes. Notably, some of these miRNAs (miR-150 and miR-155) were previously identified in circulating miRNA5 and plasma-derived exosomes from CLL patients.6 In addition, abundant miRNAs (miR-21, miR-146a) have been shown to regulate the induction of aberrant stromal cells. Exosome miRNAs were shown to transfer to MSCs and retain their functional activity. The exosome proteome was enriched in cell membrane, cytoskeletal, antiapoptotic, and angiogenic proteins. Similar to miRNAs, exosome proteins were also transferred to recipient murine and human stromal cells as exemplified by B-cell marker CD20.
The authors then study the changes induced in the target stromal cells. CLL exosomes induced an inflammatory phenotype in both MSC and endothelial cells linked to activated nuclear factor κB–dependent signaling. Gene expression profiling of exosome-treated stromal cells revealed transcriptional changes enriched for an “inflammatory response” with significant overlap to the hallmark proinflammatory gene signature of activated CAFs.7 Proinflammatory CAFs show increased expression of molecular markers, including α-smooth muscle actin (α-SMA).8 Paggetti et al verified the presence of high numbers of α-SMA–positive stromal cells in the CLL-TME.9 Gene expression changes alter the CAF secretome, and the authors’ study reveals increased levels of tumor necrosis factor family ligands (B-cell activating factor, A proliferation-inducing ligand), pro-inflammatory cytokines (eg, interleukin-6), chemokines (eg, CCL2, CXCL16), and proangiogenic factors (eg, hepatocyte growth factor) in subverted stromal cells. Distinct from normal tumor-suppressive stromal cells, CAFs promote tumor progression, angiogenesis, and dissemination through paracrine cross-talk and remodeling of the extracellular matrix. Functional data by Paggetti et al show increased collagenase activity, proliferation, and migration of exosome-educated stromal cells that exhibited striking cytoskeletal remodeling, stress fiber formation, and α-SMA expression (absent from healthy B cell–derived exosome-treated stromal cells). In vivo matrigel plug assays showed that CLL exosomes induce the formation of new blood vessels. Exosome-educated stromal cells showed an enhanced capacity to support CLL cell survival. Finally, promotion of tumor growth/dissemination was demonstrated using a cotransfer in vivo model (CLL cells with exosomes). These initial data demonstrate a protumorigenic effect of CLL-derived exosomes when tumor cells engage the microenvironment to proliferate and promote angiogenesis. Taken together, the authors conclude that CLL exosomes actively promote disease progression by subverting the function of stromal cells that reside in the TME which acquire features of proinflammatory CAFs (see figure). Clearly, future studies will need to examine the coevolution of tumor cells and the reprogrammed stroma including characterizing the role CAFs play in regulating cancer hallmark capabilities.
This work by Paggetti et al establishes the tumorigenic importance of CLL-derived exosomes, including the transfer of their molecular cargo to other TME cells. This contributes to increasing evidence that the ability of tumor cells to induce CAFs is a universal feature of progression in both solid8 and blood7,10 cancers. These data also provide incentive for detailed mechanistic investigation of the role exosomes play in pathogenic signaling processes6 and regulating the understudied CLL-TME. Examining the contribution of exosomes and their content in predicting response (biomarkers) to different therapeutic agents including newly targeted kinase inhibitors will be of interest. Paggetti et al show that CLL-derived exosomes act as decoys for the therapeutic antibody rituximab (anti-CD20) that highlights a potential drug escape mechanism in the TME. Continued research on TME regulation of tumor progression will provide insight into disease pathogenesis and also the exciting prospect of identifying novel targeted therapies designed to re‐educate the TME to have antitumorigenic effects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.